Psoriatic arthritis (PsA) is a chronic, inflammatory disorder that affects up to 30% of patients with psoriasis. Characterized by joint pain, skin lesions and systemic inflammation, PsA poses significant challenges for therapeutic development due to its clinical heterogeneity and lack of standardized diagnostic markers.
At GemPharmatech, we provide validated preclinical PsA mouse models that closely mirror the pathophysiology of huma disease. Our mannan/CFA-induced PsA model offers rapid disease onset and consistent phenotype, that makes it ideal for evaluating the efficacy of small molecules (e.g., JAK inhibitors), and biologics, including in humanized mouse strains.
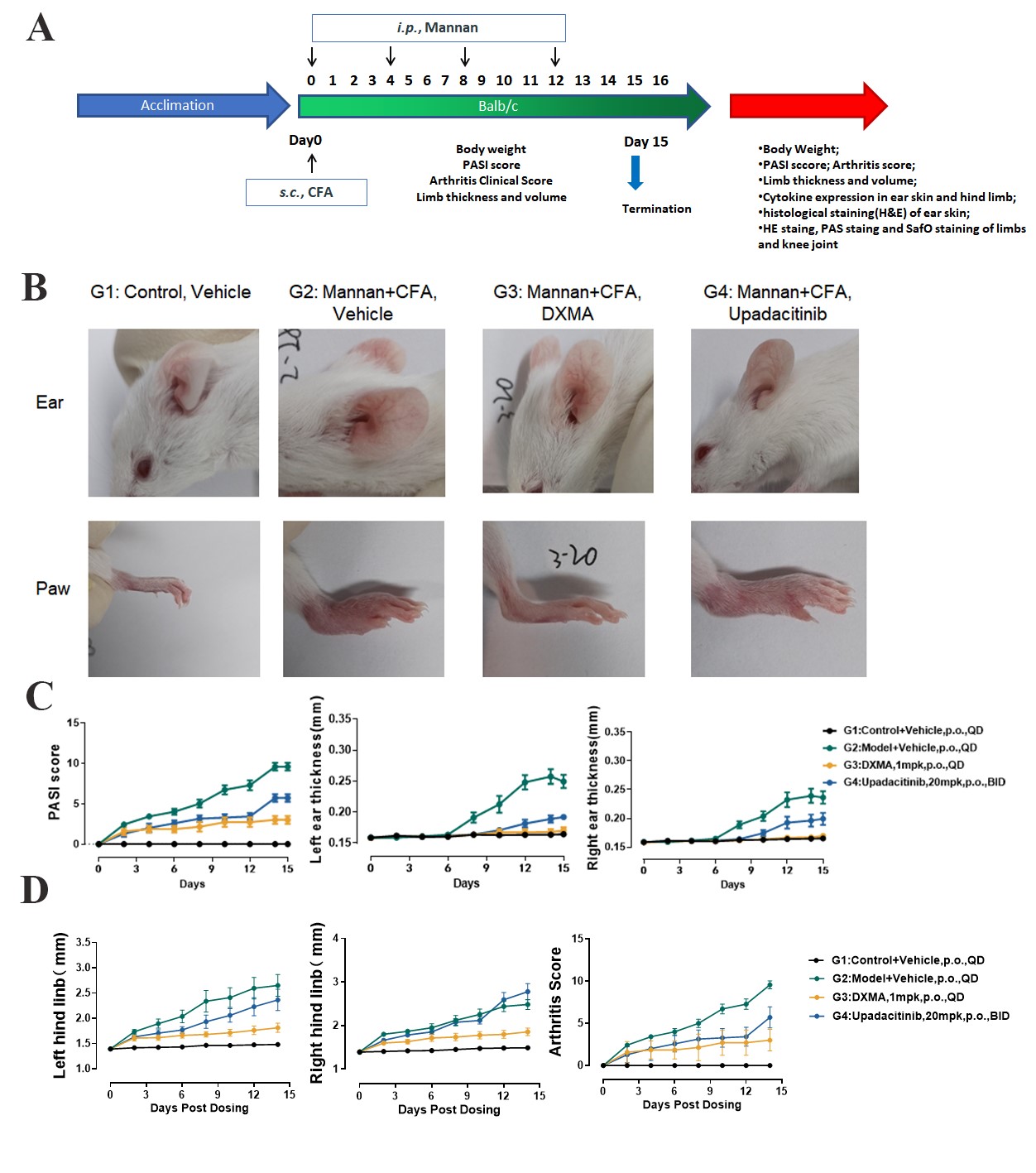
Fig.1 Pharmacodynamic data of DXMA and Upadacitinib in a psoriatic arthritis model
The study design is shown in Figure 1A. In the mannan-induced PsA model, female BALB/c mice (12-13 weeks) received intraperitoneal mannan injections (every 4 days) combined with a single subcutaneous Complete Freund’s Adjuvant (CFA) injection into the hind paw at day 0. On day 15, histopathology analysis of ear and paw tissues were conducted through HE staining and inflammation scoring. Representative photographs of mouse ears and joints are shown in Figure 1B. Compared with the model control group, the thickness of skin in mice treated with DXMA and Upadacitinib was significantly reduced (Figure 1C). Compared with the model control group, the arthritis score in mice treated with DXMA and Upadacitinib was significantly decreased (Figure 1D).
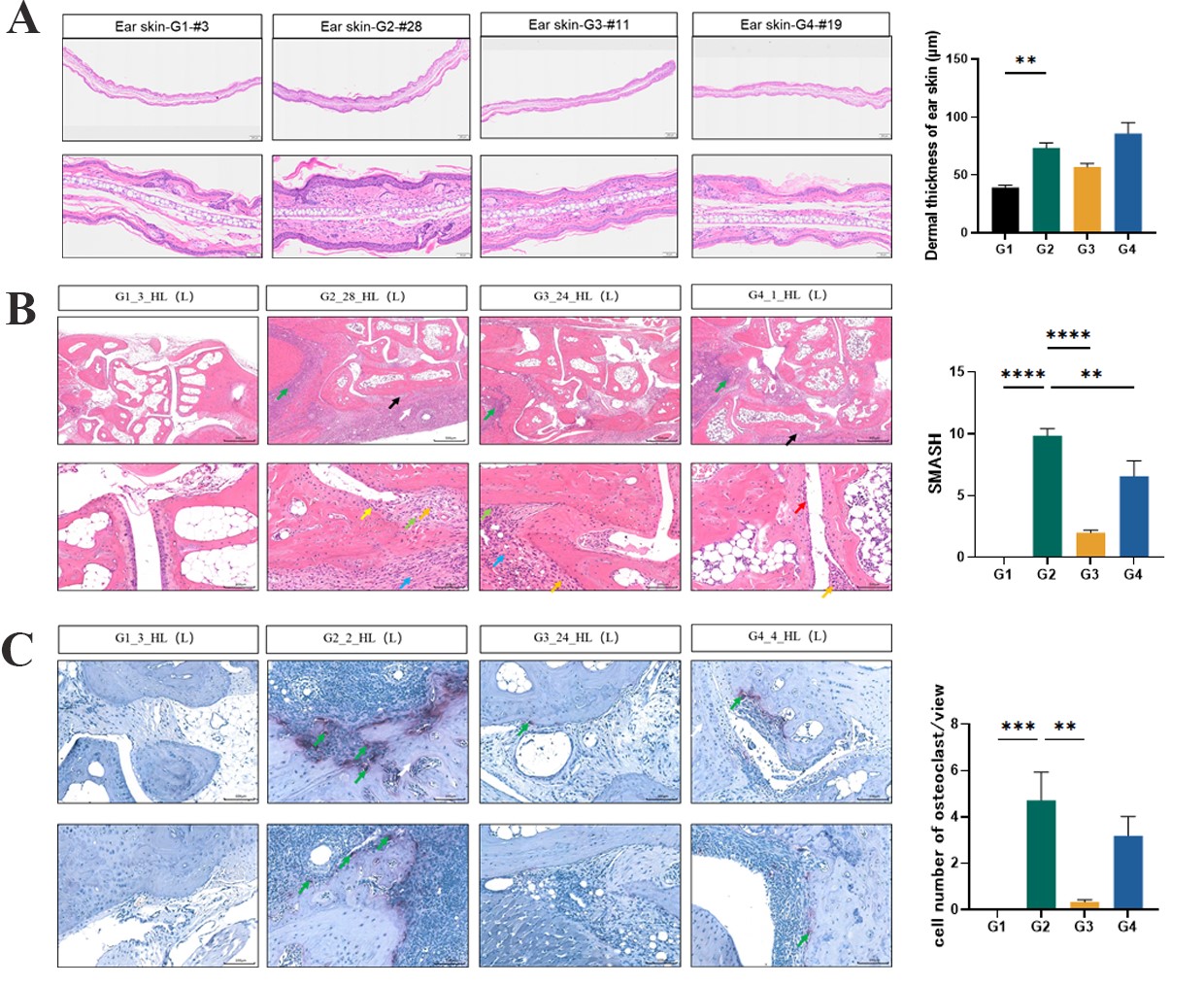
Fig. 2 Histopathological staining results of ears (HE Staining) and joints (HE and TRAP Staining)
Compared to the healthy control group, the dermal thickness of the ear skin (Figure 2A), the arthritis pathological score (Figure 2B), and the number of osteoclasts (Figure 2C) were notably increased in the model group. Treatment with DXMA and Upadacitinib resulted in a reduction of the arthritis pathological scores.