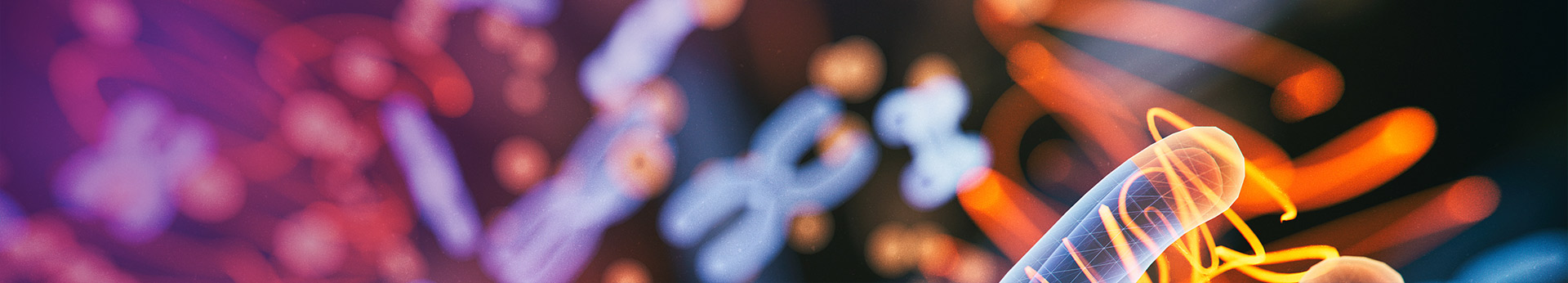B6-Trex1-KO mice
TREX1 (DNase III) is the primary 3'-5' DNA exonuclease in mammalian cells, playing a key role in cell death and genomic DNA degradation. The B6-Trex1-KO mice, which lack the Trex1 gene, exhibit TREX1 dysfunction leading to DNA accumulation and activation of autoimmune responses. These mice show elevated serum anti-dsDNA levels and develop multi-organ inflammatory phenotype. This model can be used to evaluate the potential pathogenic mechanisms of TREX1-related signaling pathways in SLE, providing a valuable tool for studying the pathogenesis of innate immunity-mediated autoimmune diseases and assessing potential therapeutic strategies for SLE.
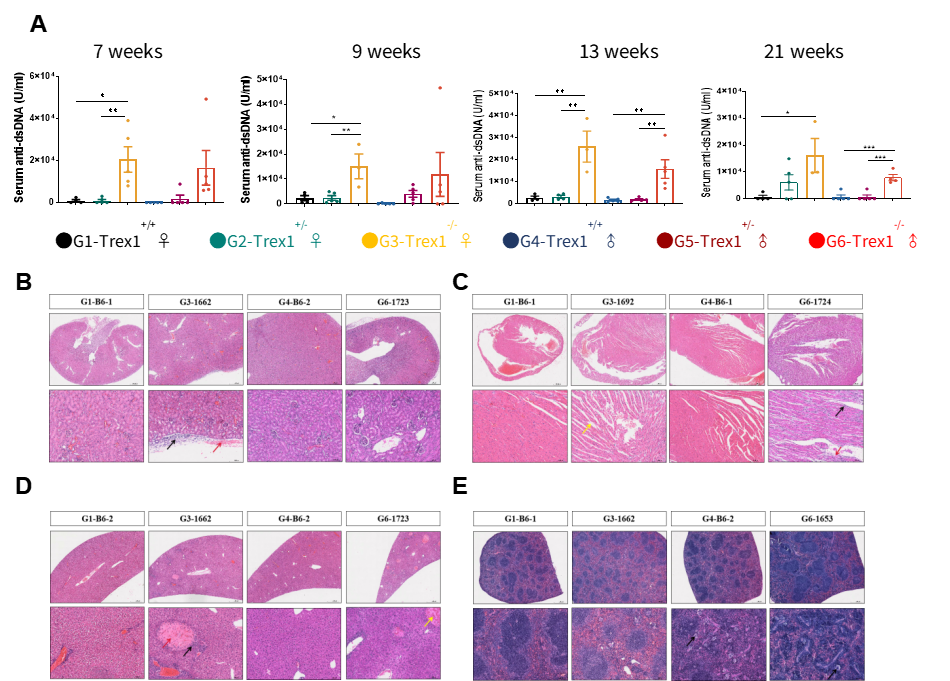
Fig.1 B6-Trex1-KO mice
In both female and male mice aged 7 to 21 weeks, serum anti-dsDNA levels were elevated in B6-Trex1-KO mice compared to control B6 mice. This result aligns with the previously described autoimmune phenotype of TREX1-deficient mice, where genomic DNA accumulation triggers autoimmune responses characteristic of SLE pathogenesis. Increased infiltration of inflammatory cells was observed in various tissues and organs of B6-Trex1-KO mice (6 weeks old).
B6-hBAFF mice
BAFF, also known as B-cell-activating factor of the tumour-necrosis-factor family (TNFSF13B), is a key cytokine in the TNF superfamily. BAFF is frequently overexpressed in SLE patients and plays a critical role in disease pathogenesis by promoting the expansion of autoreactive B cells. GemPharmatech has generated transgenic mice overexpressing human BAFF using gene editing technology. Compared to wild-type mice, these transgenic mice exhibit elevated IgG, IgA, IgM, and anti-dsDNA antibody levels, with antibody titers increasing with age. Additionally, these mice present with elevated renal pathological indices, making this model suitable for evaluating the efficacy of drugs targeting B cells and the B cell BTK pathway in SLE and lupus nephritis.
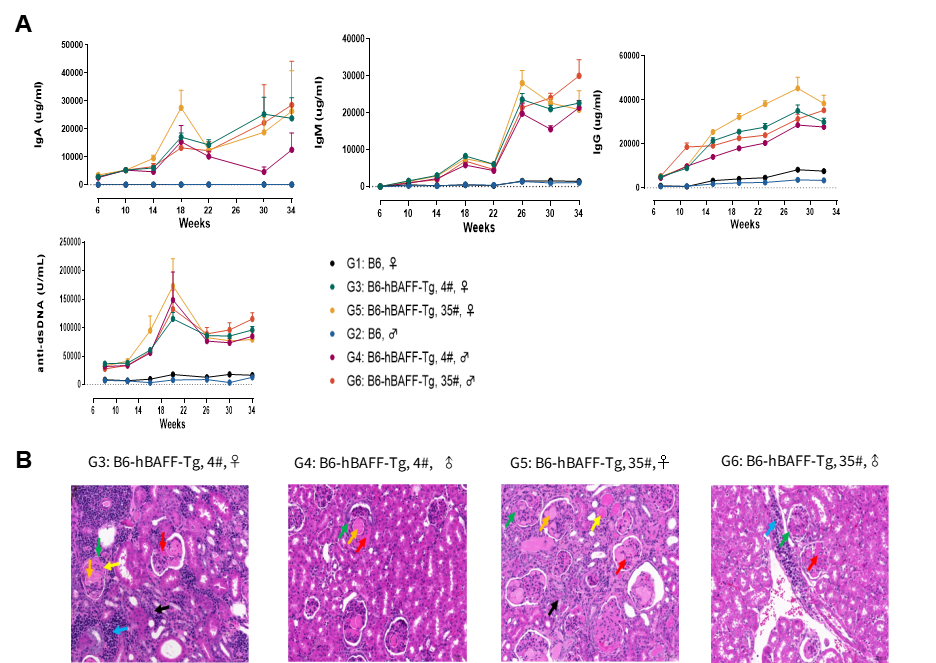
Fig.2 B6-hBAFF Mice
The concentrations of IgA, IgM and IgG in the serum of B6-hBAFF mice continuously increased, significantly higher than those of the control group mice. The anti-dsDNA level continuously increased and obvious kidney lesions appeared at 25 weeks of age. B6-hBAFF Mice Show Lupus Nephritis , glomerular enlargement and renal capsule stenosis (↑). Immune complex deposition is visible in subendothelial along with segmental fibrinoid necrosis (↑), and crescent formation (↑). The surrounding renal tubules show atrophy (↑), and lymphocyte infiltration (↑).
BALB/c-Tlr7-p.Y264H mice
Toll-like receptor 7 (TLR7) directly recognizes ligands such as single-stranded RNA or nucleic acid analogs, binds to specific recruitment proteins, and activates regulatory factors including NF-κB, MAPK, and IFN, thereby participating in both innate and adaptive immune responses. Enhanced TLR7 signaling has been detected in most systemic lupus erythematosus (SLE) patients, indicating its critical role in SLE pathogenesis. GemPharmatech has developed the BALB/c-Tlr7-p.Y264H mouse model using gene editing technology, which introduces a tyrosine-to-histidine mutation at position 264 (p.Y264H) in the Tlr7 gene of BALB/c mice. At 16 weeks of age, these mice spontaneously develop SLE-like disease with elevated disease-related markers. This model highly recapitulates clinical TLR7-mutant SLE, facilitating exploration of disease mechanisms and providing a suitable animal model for the development of novel therapeutic strategies targeting SLE.
Fig3 BALB/c-Tlr7-p.Y264H mice
Schematic diagram of Tlr7 gene mutation strategy in BALB/c-Tlr7-p.Y264H mice. HE staining assessment of glomerular atrophy (green arrowheads) and renal tubular epithelial cell degeneration or regeneration with nuclei protruding into the tubular lumen (red arrowheads).