Alms1 is a ubiquitous protein that is essential for normal primary ciliary function. Although its specific regulation mechanism has not been fully elucidated, the present reports indicate that absence of Alms1 protein will promote nutrient absorption and increase food Intake. Mutations of the Alms1 gene are associated with Alström syndrome, an autosomal recessive disorder of childhood obesity characterized by type 2 diabetes, dyslipidemia, low growth hormone levels, hypothyroidism, infertility, and abnormalities of the kidneys, heart, and liver. Mice with Alms1 gene deletion are susceptible to insulin resistance, obesity, diabetes and Metabolic Dysfunction-Associated Steatohepatitis (MASH).
Our Alms1 Knockout model carries a deletion of an 11bp sequence of exon 8 of the mouse Alms1 gene, resulting in early termination of translation. B6-Alms1-del mice develop obesity, diabetes and MASH spontaneously providing a valuable animal model for studying mechanisms underlying metabolic syndromes (MetS) and evaluating the efficacy of anti-MetS and anti-MASH agents.
Example Data
1. Growth curve, body fat ratio and food intake
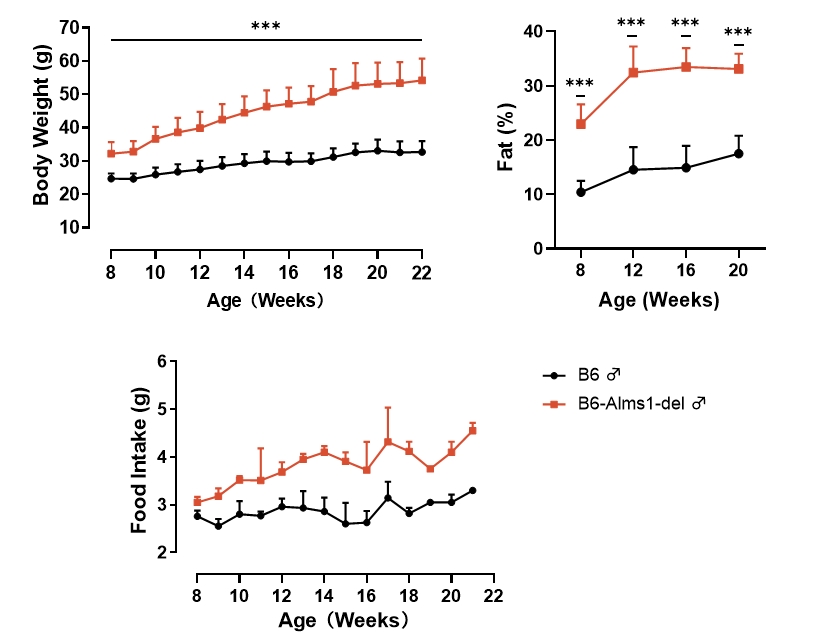
Fig 1. B6-Alms1-del male mice develop spontaneous obesity
Both B6 (n=6-18♂) and B6-Alms1-del (n=6-18♂) mice were fed standard diet (6% fat). B6-Alms1-del males consumed more food than B6 control males and had developed a spontaneous obesity by 8-week-old, as evidenced by significantly higher body weights and body fat ratios than those of the B6 control males, which continued to increase with increasing age. (Data were presented as Mean±SD, *** indicated p<0.001)
2. Blood glucose, insulin levels and oral glucose tolerance test
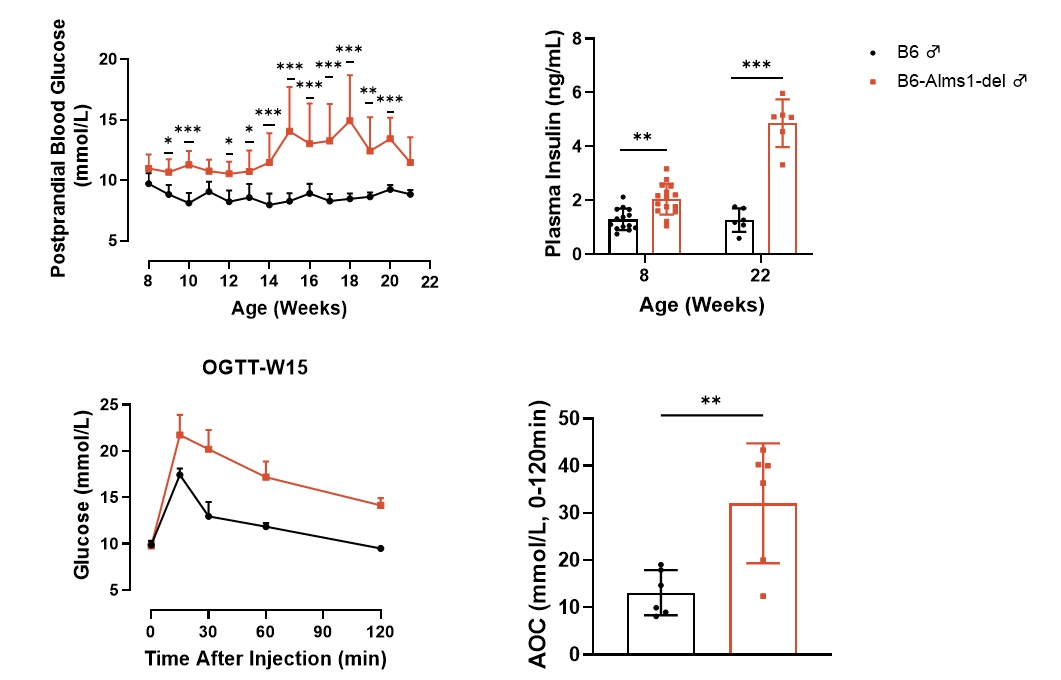
Fig 2. B6-Alms1-del male mice developed type 2 diabetes spontaneously
Both B6 (n=6-18 ♂) and B6-Alms1-del (n=6-18 ♂) mice were fed standard chow. B6-Alms1-del males developed spontaneous hyperglycemia as well as hyperinsulinemia, and symptoms tended to increase with age. The mice were subjected to OGTT at the age of 15-weeks, and glucose tolerance was significantly impaired in B6-Alms1-del males compared to B6 controls. (Data are presented as Mean ± SD, *, **, *** indicated p < 0.05, p < 0.01, p < 0.001, respectively)
3. Blood lipid and transaminase levels
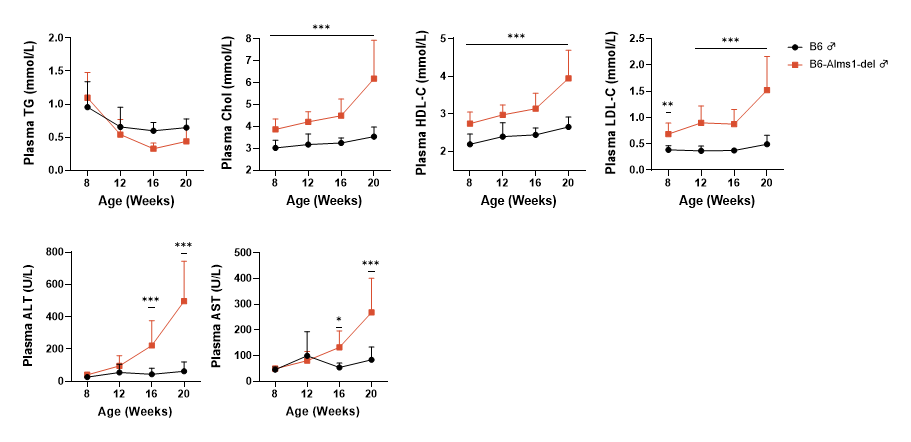
Fig 3. B6-Alms1-del male mice developed hypercholesterolemia spontaneously
Both B6 (n=6-18♂) and B6-Alms1-del (n=6-18♂) mice were fed standard diet. The plasma levels of lipids (triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol) and aminotransferases (glutamic pyruvic transaminase and glutamic oxaloacetic transaminase) were measured by automatic blood biochemistry at the age of 8, 12, 16, and 20 weeks, and the cholesterol, HDL-C and LDL-C were significantly higher in the B6-Alms1-del mice when compared with B6 male mice at the age of 8 weeks and the difference was amplified with age. Plasma aminotransferase levels in B6-Alms1-del males were significantly higher than those in B6 males from 16-week-old, and the difference further increased with age. (Data are presented as Mean±SD, *, *** indicated p<0.05, p<0.001, respectively)
4. Liver mass and lipid levels
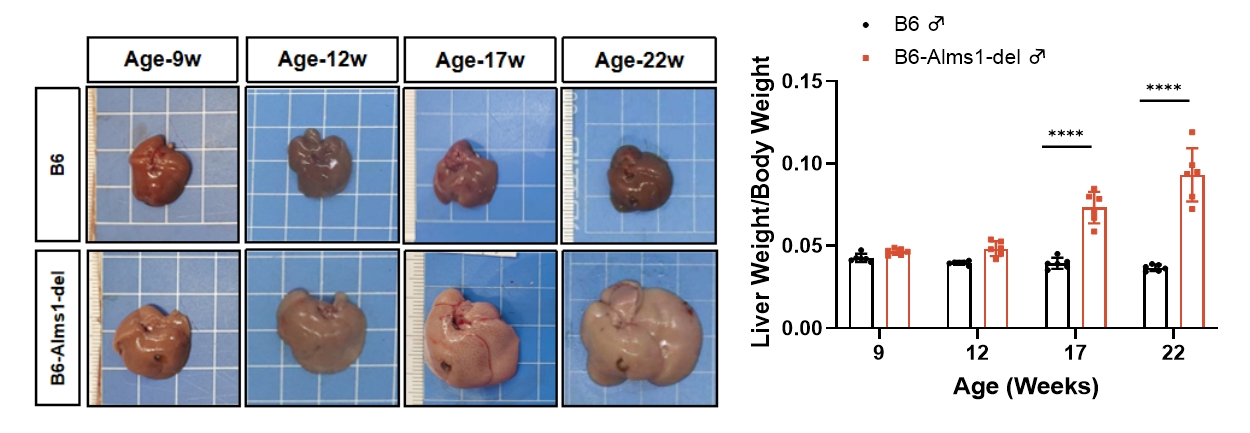
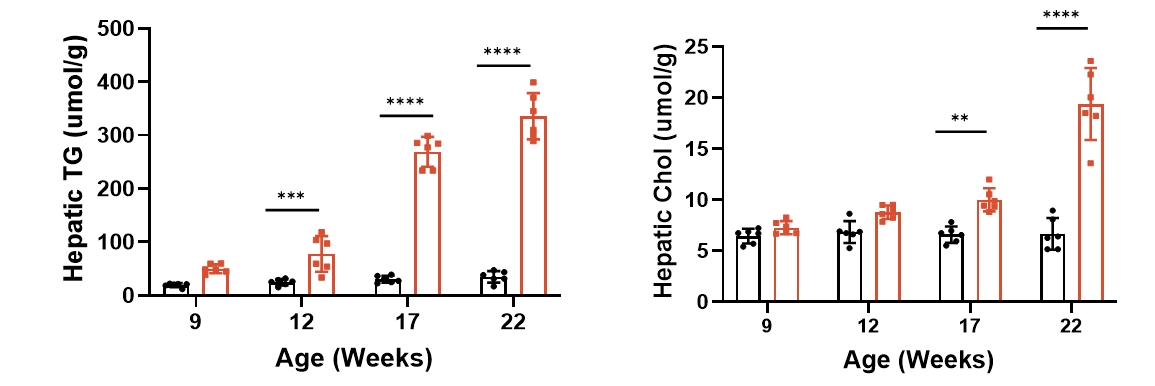
Fig 4. B6-Alms1-del male mice developed fatty liver spontaneously
Both B6 (n=6♂) and B6-Alms1-del (n=6♂) mice were fed with standard chow. The liver mass and volume of B6-Alms1-del male mice increased with age and showed a distinct fatty liver appearance (whitish and rough surface). The liver lipid content (TG and TC levels) of mice at the age of 8, 12, 17 and 22 weeks was measured by automated blood biochemistry in the liver lysate, and the liver triglyceride and cholesterol contents of B6-Alms1-del males were significantly higher than those of B6 males from the age of 12 to 17 weeks, and the differences further amplified with the age. (Data were presented as Mean±SD, **, **** indicated p<0.01, p<0.0001, respectively)
5. Hepatic H&E staining and mRNA levels of inflammatory factors
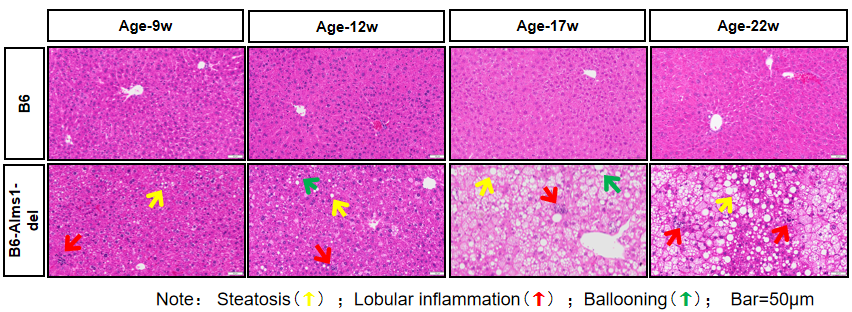
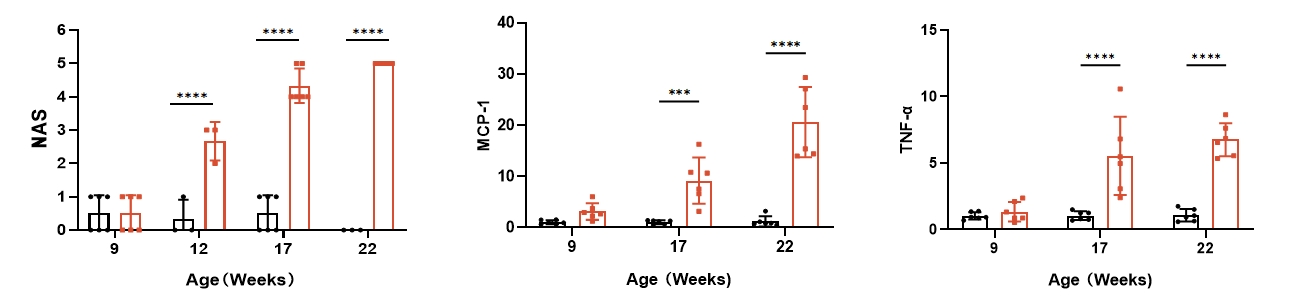
Fig 5. B6-Alms1-del male mice developed fatty liver and hepatitis spontaneously
Both B6 (n=3-6♂) and B6-Alms1-del (n=3-6♂) mice were fed with chow diet. Liver sections were stained with H&E to evaluate the NAS (NAFLD Activity Score). Compared with B6 males, B6-Alms1-del males showed hepatic steatosis, lobular inflammation, and ballooning from the age of 12 weeks onward, and such lesions progressed with age. The mRNA expression levels of inflammatory factors MCP-1 and TNF-α in the livers of B6-Alms1-del male mice were significantly elevated from the age of 17 weeks compared with that in the B6 male mice. (Data are presented as Mean±SD, ***, **** indicated p<0.001, p<0.0001, respectively)
6. Hepatic PSR staining, α-SMA IHC staining and mRNA levels of fibrosis genes
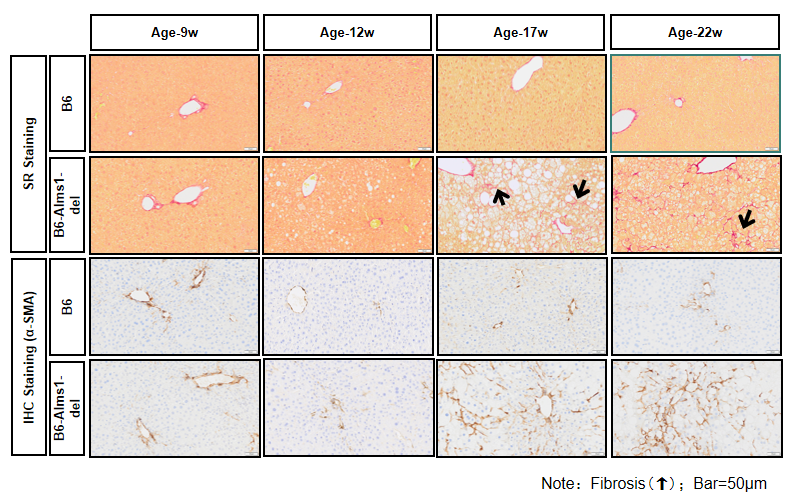
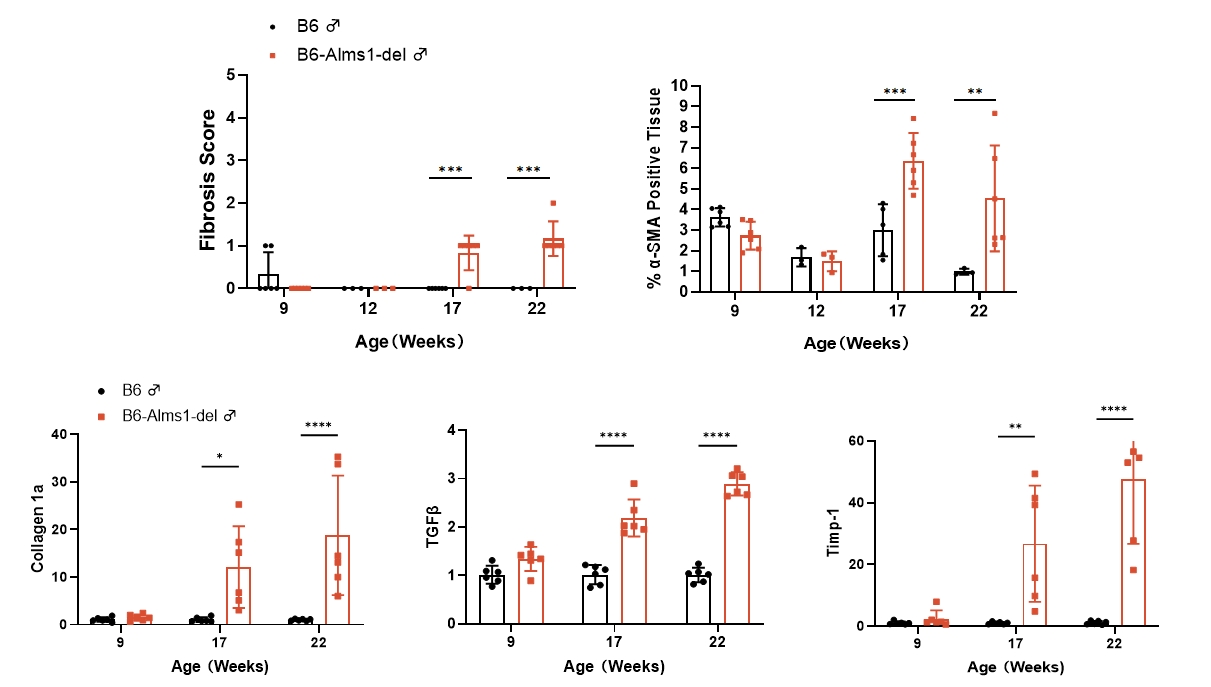
Fig 6. B6-Alms1-del male mice developed hepatic fibrosis spontaneously
Both B6 (n=3-6♂) and B6-Alms1-del (n=3-6♂) mice were fed with chow diet. The degree of hepatic fibrosis in the mice was evaluated by PSR staining, fibrosis scoring, quantitative analysis of α-SMA-positive signals, and mRNA expression levels of fibrosis-related genes in the liver. The results showed that compared with B6 male mice, B6-Alms1-del male mice developed hepatic fibrosis from the age of 17 weeks, and the degree of fibrosis progressed with age. (Data were presented as Mean±SD, *, **, ***, **** indicated p<0.05, p<0.01, p<0.001, p<0.0001, respectively)
7. Renal function and bone density
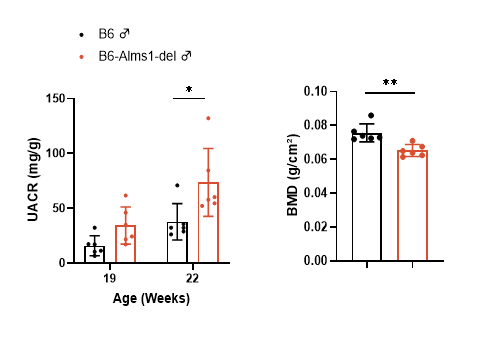
Fig 7. B6-Alms1-del male mice developed renal injury and bone loss spontaneously
Both B6 (n=6♂) and B6-Alms1-del (n=6♂) mice were fed with chow diet. UACR was calculated by collecting spot urine and measuring urinary albumin and creatinine levels; UACR levels were elevated in B6-Alms1-del males compared with B6 males, and the difference was significant at 22 weeks of age. Changes in bone mass were measured by DEXA in mice, and bone density was significantly lower in B6-Alms1-del males compared with B6 males at the age of 20 weeks. (Data presented as Mean ± SD, *, ** indicated p < 0.05, p < 0.01, respectively)
8. Pharmacodynamic testing programs
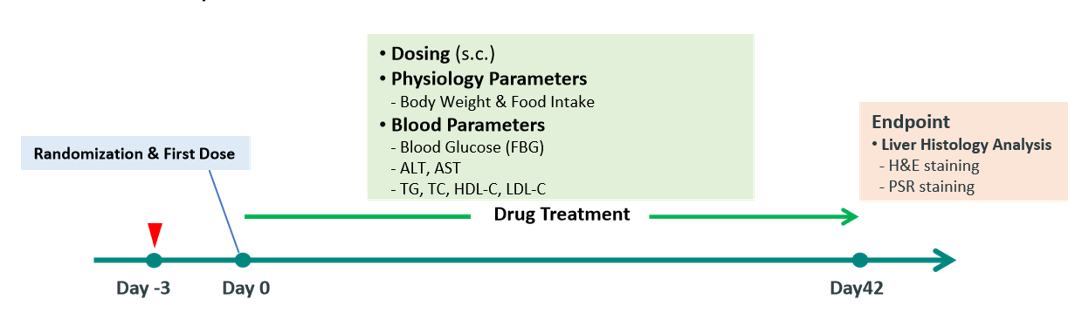
Fig 8. Typical study protocol for MASH pharmacodynamic studies in B6-Alms1-del male mice
Male C57BL/6JGpt mice and B6-Alms1-del mice at 16 weeks of age were randomized by plasma ALT level and body weight, and then received twice weekly treatment of vehicle, 30nmol/kg Tirzepatide or 30nmol/kg Efruxifermin subcutaneously for 6 weeks. Body weight, food intake, liver enzymes, fasting blood glucose and blood lipids were monitored in life and the histopathological analysis of liver and liver lipid content were performed at study end.
9. Drug efficacy study - body weight, food intake, blood glucose and lipid levels
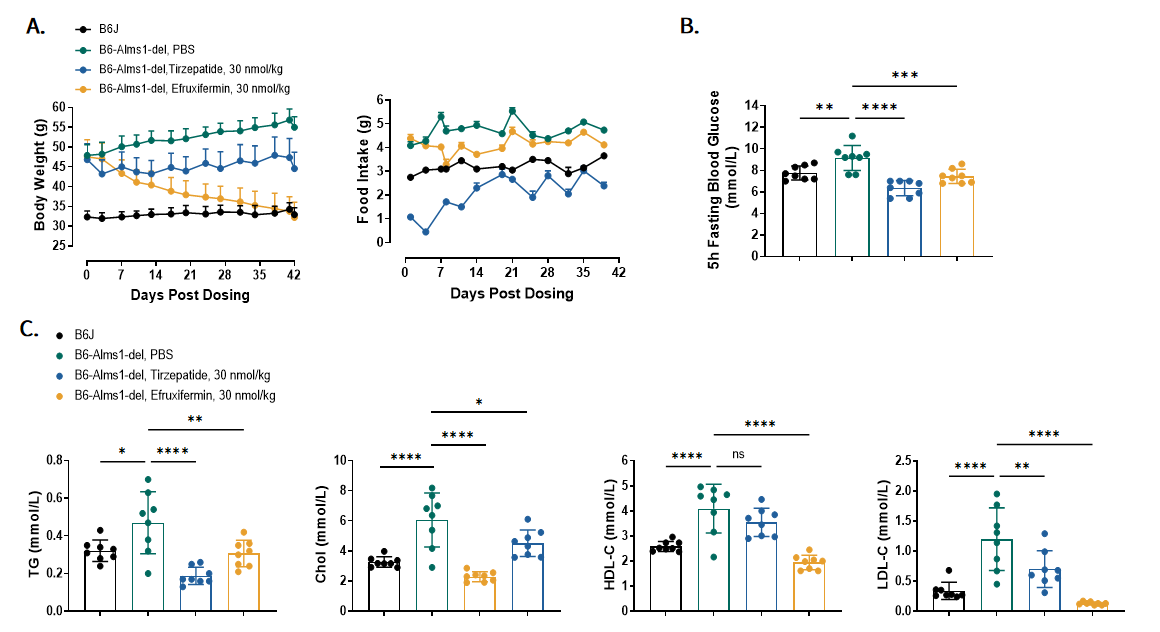
Fig 9. Tirzepatide and Efruxifermin treatment improved metabolic syndrome of B6-Alms1-del mice
A. Body weight and 24h food intake change across six weeks. B. Fasting blood glucose was measured at Day17. C. Blood lipid concentration was measured after six weeks. (Data are presented as Mean ± SD, *, **, ***, **** indicated p < 0.05, p < 0.01, p < 0.001, p < 0.0001 respectively)
10. Drug efficacy study - transaminases, liver mass and lipid levels
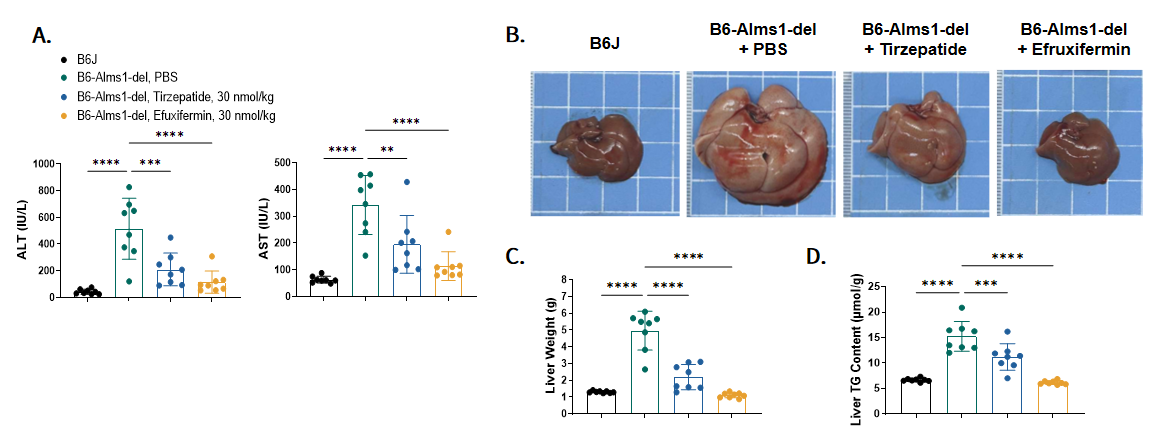
Fig 10. Tirzepatide and Efruxifermin treatment significantly reduced liver injury, liver hypertrophy and liver lipid content of B6-Alms1-del mice
A.Plasma liver enzymes ALT and AST detected after six weeks of treatment. B and C. Liver morphology of each group at the endpoint. D. Liver triglyceride content detected at the endpoint. (Data are presented as Mean ± SD, **, ***, **** indicated p < 0.01, p < 0.001, p < 0.0001 respectively)
11. Drug efficacy study - hepatic H&E and PSR staining
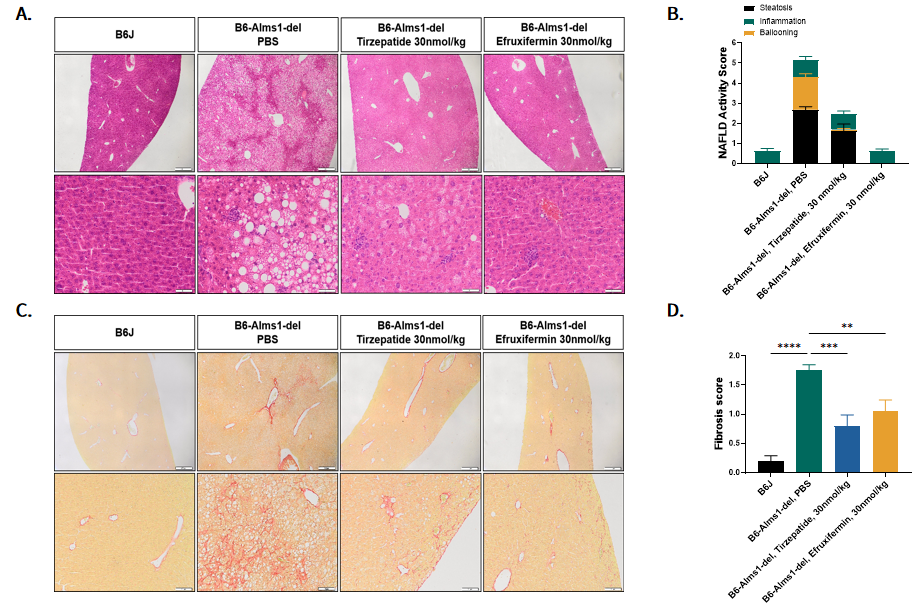
Fig 11. Tirzepatide and Efruxifermin treatment decreased NAFLD activity score and improved liver fibrosis of B6-Alms1-del mice.
A and B. H&E staining and NAFLD Activity Scores, Scale bar = 500 μm (upper panel), 50 μm(bottom panel). C and D. Sirius Red staining and fibrosis scores. Scale bar = 500 μm (upper panel), 100 μm(bottom panel). (Data are presented as Mean ± SD, **, ***, **** indicated p < 0.01, p < 0.001, p < 0.0001 respectively)
12. High-fat induction - body weight change
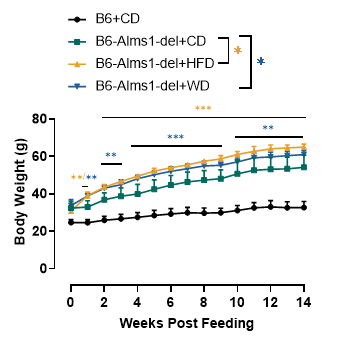
Fig 12. 60% HFD or western diet (40% fat) induction significantly increased body weight of B6-Alms1-del mice
B6-Alms1-del mice had significantly higher body weights induced by 60% high-fat diet or western diet compared to chow diet feeding, but there was no difference in weight gain induced by the two different diets. (Data are presented as Mean±SD, **,*** indicated p<0.01, p<0.001, respectively)
13. High-fat induction - blood lipid and transaminase levels
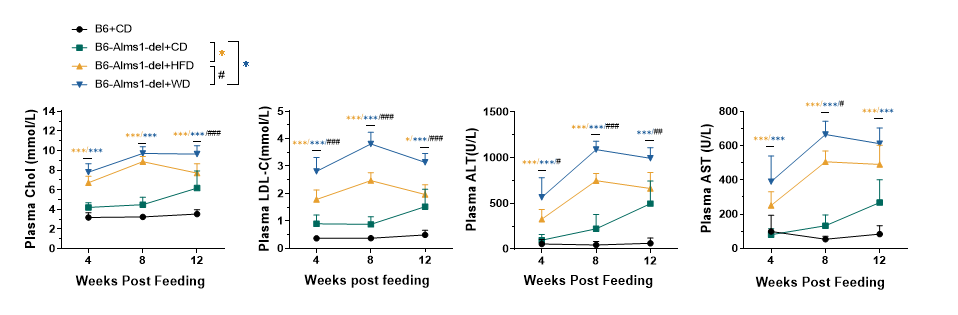
Fig 13. 60% HFD or WD induction significantly increased plasma cholesterol and transaminase levels of B6-Alms1-del mice
Plasma cholesterol and aminotransferase levels were significantly higher in B6-Alms1-del fed with 60% high-fat diet or Western diet compared to chow diet feeding, and plasma cholesterol and aminotransferase levels were significantly higher in western diet feeding than high-fat diet feeding. (Data are presented as Mean±SD, *,*** indicated p<0.05, p<0.001 respectively; #, ##, ### indicated p<0.05, p<0.01, p<0.001, respectively)
14. High-fat induction - blood glucose, insulin levels and oral glucose tolerance tests
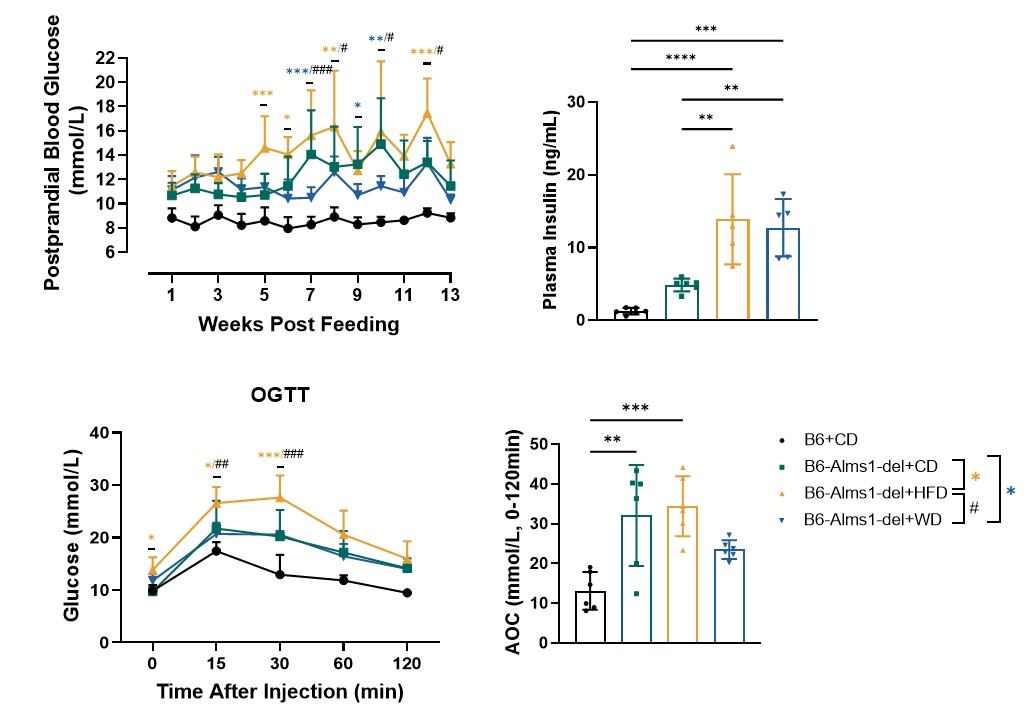
Fig 14. 60% HFD induction significantly increased postprandial blood glucose and plasma insulin levels of B6-Alms1-del mice, while impaired glucose tolerance
With the Induction of 60% high-fat diet, postprandial blood glucose and plasma insulin levels of B6-Alms1-del mice were significantly elevated and glucose tolerance was impaired compared to mice maintained on standard 6% fat chow (CD). With the induction of western diet, plasma insulin levels of B6-Alms1-del mice were significantly elevated, but there was no difference in postprandial blood glucose and glucose tolerance compared to chow diet feeding. (Data are presented as Mean±SD, *, **, *** indicated p<0.05, p<0.01, p<0.001, respectively; #, ##, ### indicated p<0.05, p<0.01, p<0.001, respectively)
15. High-fat induction - hepatic H&E staining
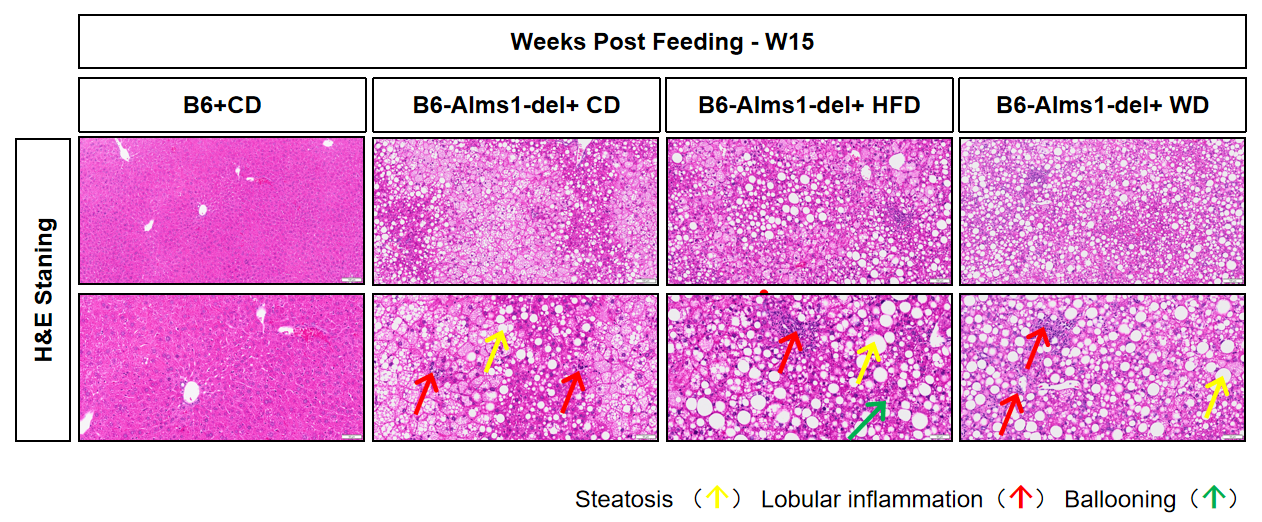
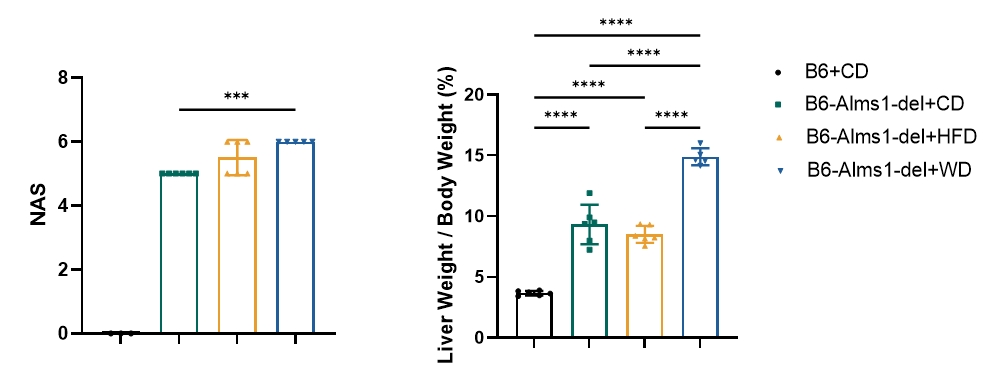
Fig 15. WD induction significantly increased NAFLD activity score and liver mass of B6-Alms1-del mice
Western diet feeding induced significantly higher NAFLD activity score and liver mass in B6-Alms1-del mice when compared with chow diet feeding. While there was no difference between 60% high-fat diet and chow diet in these parameters. (Data are presented as Mean ± SD, ***, **** indicated p < 0.001, p < 0.0001, respectively)
16. High-fat induction - hepatic PSR and α-SMA IHC staining
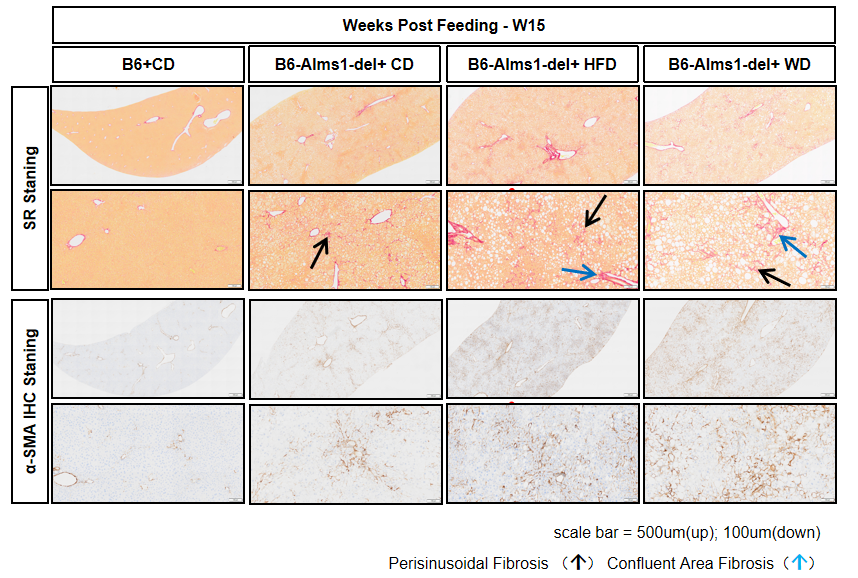
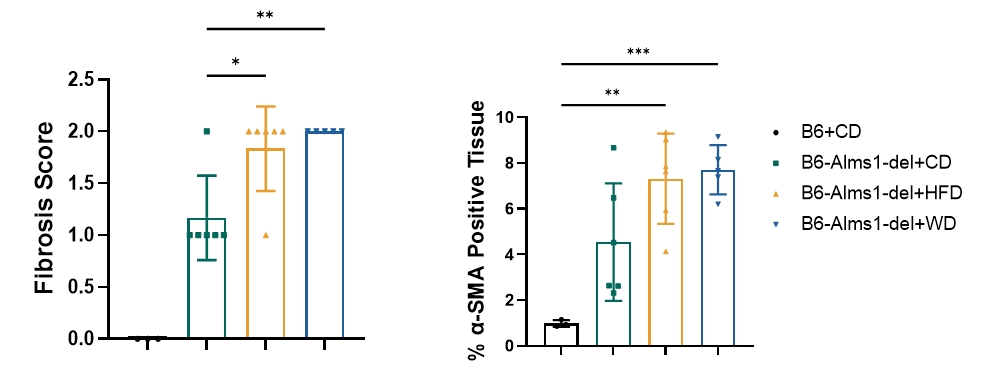
Fig 16. 60% HFD or WD induction significantly increased hepatic fibrosis of B6-Alms1-del mice
60% high-fat diet or western diet feeding exacerbated hepatic fibrosis with significant perivascular and interstitial fibrosis. (Data are presented as Mean±SD, *, **, *** indicated p<0.05, p<0.01, p<0.001, respectively)
References
1. Scott L. Friedman, et al. Mechanisms of MASLD development and therapeutic strategies. Nat Med. 2018 Jul; 24(7):908-922.
2. Todor Arsov, et al. Fat Aussie—A New Alström Syndrome Mouse Showing a Critical Role for ALMS1 in Obesity, Diabetes, and Spermatogenesis. Molecular Endocrinology, Volume 20, Issue 7, 1 July 2006, Pages 1610–1622
3. Haczeyni F, et al. Obeticholic Acid Improves Adipose Morphometry and Inflammation and Reduces Steatosis in Dietary but not Metabolic Obesity in Mice. Obesity (Silver Spring). 2017 Jan; 25 (1):155-165.
4. Mridha AR, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. Send to J Hepatol. 2017 May; 66(5):1037-1046.