Immune checkpoint receptors play an important role in maintaining immune balance and regulating the occurrence and development of immune diseases. In the process of developing new antibody drugs targeting immune checkpoints, the binding properties of antibodies with antigen targets are one of the key methods to evaluate the function of antibodies. Antibody binding assays are used to evaluate the binding ability of antibody drugs with target-positive cells and target humanized mouse cells in vitro. These assays also compare the tested articles with the positive drug to provide references for efficacy studies in vivo.
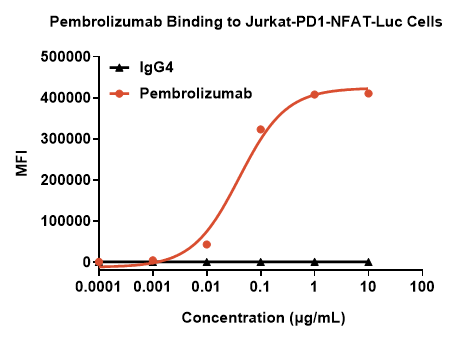
Case: The Binding Assay of Pembrolizumab with PD-1 Expressing Cells at Different Concentrations.
Fig. Flow cytometry analysis of Pembrolizumab monoclonal antibody (mAb) and PD-1 expression on immune cells under various conditions.
GemPharmatech Advantages
Extensive library of target cells (300+ cancer cell lines and humanized cell lines) and qualified BSL-2 level laboratories.
Providing comparator antibody therapy for common viral infections for free.
In addition to the standard cell lines, new modified cell lines can be constructed to meet client project demands, ensuring a smooth progression of experiments.
Extensive project experience.
Satisfying the regulatory requirements for new drug application submissions.
Strict laboratory data storage standards ensure experiment traceability.