PD1 (programmed cell death 1), a type I transmembrane glycoprotein in the immunoglobulin and CD28 superfamily, is an essential immunosuppressive molecule. Immunoregulation targeted at PD1 is crucial for anticancer, anti-infectious, anti-autoimmune, and post-transplantation rejection reactions. Several studies have demonstrated that the expression of PKL1 increases on the surface of tumor cells in the tumor microenvironment. Simultaneously, when binding to PD1 on activated T-cells, the ligand is able to transmit negative regulatory signals, leading to apoptosis and functional failure of tumor antigen-specific T-cells, as well as the subsequent suppression of immune response and escape of tumor cells. The blockage of the PD1/PDL1 signaling pathway has become a classic method for immunotherapy of tumors [1-3].
Development Strategy
GemPharmatech has successfully developed single-target humanized PD1 mouse models, including single-target humanized mouse models BALB/c-hPD1 and C57BL/6-hPD1, and double-humanized mouse models BALB/c-hPD1/hPDL1 and C57BL/6- hPD1/hPDL1, by replacing the extracellular parts of PD1 of BALB/c and C57BL/6 mice with corresponding human segments without impairing the integrity of the cytoplasmic region of mouse PD1. PD1 humanized mouse models are ideal animal models for efficacy evaluation and safety evaluation of human PD1 inhibitors.
1. BALB/c-hPD1: KEYTRUDA®+ ENT Combination Drug Efficacy Test
The anti-human PD1 antibody KEYTRUDA® (Pembrolizumab) and Entinostat (ENT), small-molecule inhibitors of HDAC, were investigated in BALB/c-hPD1 mice that had been subcutaneously implanted with the CT26.WT tumor cell line.
KEYTRUDA® (Pembrolizumab): an anti-human PD1 antibody antitumor drug marketed by Merck Sharp & Dohme.
Entinostat (ENT): a selective HDAC1 inhibitor that acts on HDAC1, HDAC2 and HDAC3, developed by Syndax.
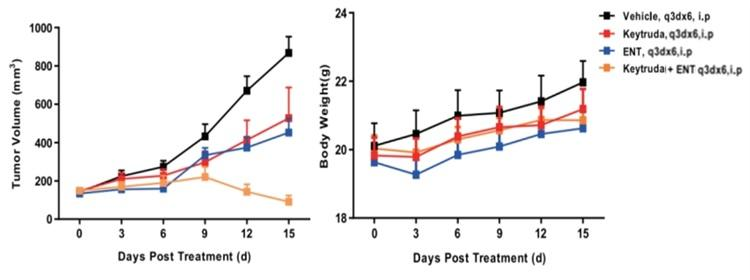
Left Figure: Mouse tumor growth curve. Right Figure: Mouse body weight curve.
In vivo efficacy test of combo therapy in a model of BALB/c-hPD1 mice subcutaneously inoculated with CT26.WT. Colon cancer cells CT26.WT in the logarithmic growth phase were subcutaneously inoculated into 6-8 week-old humanized BALB/c-hPD1 mice. When tumors reached an average volume of about 100 mm3, the mice were randomized to Vehicle (Control) Group, KEYTRUDA® Monotherapy Group, ENT Monotherapy Group, or KEYTRUDA® + ENT Combination Therapy Group (n = 7-9) and treated with the corresponding drugs (KEYTRUDA®: 10 mpk; ENT: Entinostat, 20 mpk). The drugs were administered every three days for a total of 6 doses. The data are presented as Mean ± SEM.
Results: KEYTRUDA® or Entinostat monotherapy had an inhibitory effect on tumor growth (TGI = 45.3% in the KEYTRUDA® Treatment Group, TGI = 44.6% in the ENT Treatment Group). When combined with Entinostat, KEYTRUDA® had a more powerful tumor-inhibiting activity than single drug administration (TGI = 89.5%)(Left Figure). The four groups of mice showed similar body weight changes (Right Figure).
These findings demonstrate the BALB/c-hPD1 mouse is an ideal model for evaluating the in vivo efficacy of anti-human PD1 antibodies and the other drug combination therapies.
2. C57BL/6-hPD1: KEYTRUDA® drug efficacy test
The tumor inhibitory efficacy of KEYTRUDA® (Pembrolizumab) at different doses was tested in a model of B6-hPD1 mice subcutaneously inoculated with MC38 tumor cell line.
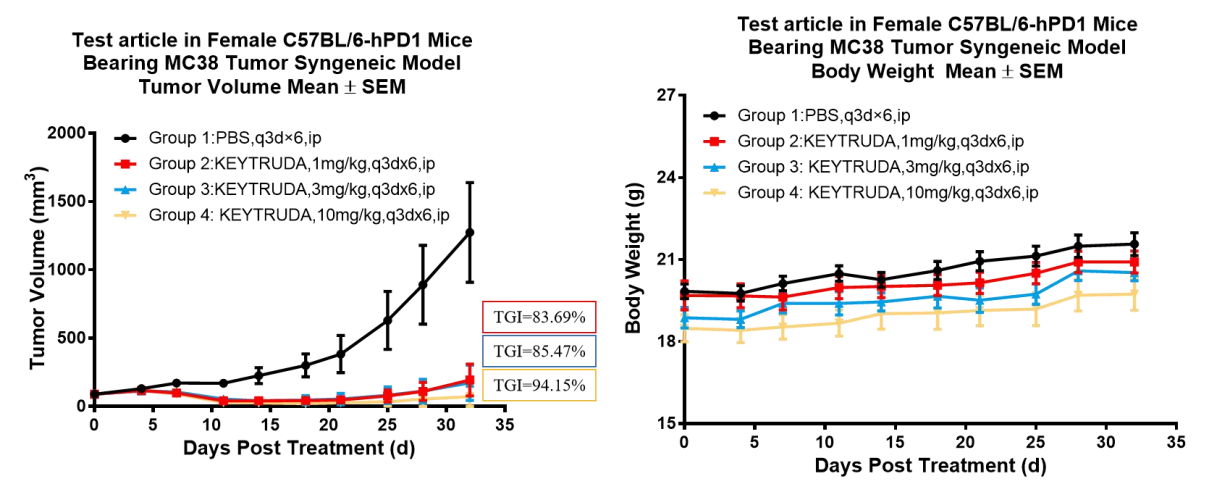
Left Figure: Mouse tumor growth curve. Right Figure: Mouse body weight curve.
Murine colon cancer cells MC38 in the logarithmic growth phase were subcutaneously inoculated into 6-8 week-old humanized C57BL/6-hPD1 mice. When tumors reached an average volume of about 100 mm3, the mice were randomized to Vehicle (control) Group, KEYTRUDA® 0.5 mpk Group, KEYTRUDA® 1 mpk Group, KEYTRUDA® 5 mpk Group and KEYTRUDA® 10 mpk Group (n = 8) and treated with the specified drugs at corresponding dose levels. The drugs were administered every three days for a total of 6 doses. The data are presented as Mean ± SEM.
Results: The tumor growth inhibition (TGI) of KEYTRUDA® varied with dose; the most significant tumor inhibitory effect was seen in the 10 mpk Group (TGI = 79.7 %); tumor volumes were similar to the 0.5 mpk, 1 mpk, and 5 mpk Groups, with TGIs of 44.7%, 58%, and 56.2%, respectively (Left Figure). The five mouse groups had similar body weight changes (Right Figure).
The results show that the B6-hPD1 mouse is an ideal animal model for evaluating the efficacy of human PD1 antibodies.
3. C57BL/6-hPD1: OPDIVO® Drug Efficacy Test
The tumor inhibitory efficacy of OPDIVO® (Nivolumab), an anti-human PD1 antibody, was tested in B6-hPD1 mice that were subcutaneously inoculated with MC38 tumor cell line at different doses.
OPDIVO® (Nivolumab): an antibody human PD1 antibody antitumor drug marketed by Bristol Myers Squibb.
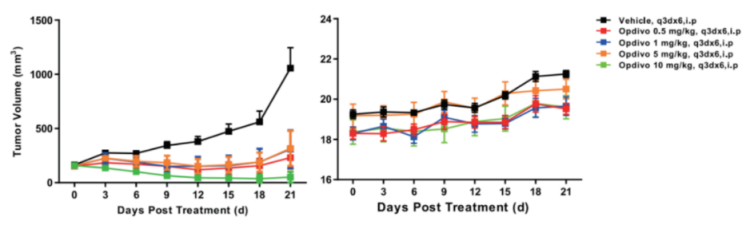
Left Figure: Mouse tumor growth curve. Right Figure: Mouse body weight curve.
Colon cancer cells MC38 in the logarithmic growth phase were subcutaneously inoculated into 6-8 week-old humanized B6--hPD1 mice. When tumors reached an average volume of about 100 mm3, the mice were randomized to Vehicle (Control) Group, OPDIVO® 0.5 mpk Group, OPDIVO® 1 mpk Group, OPDIVO® 5 mpk Group, and OPDIVO® 10 mpk Group (n = 6) and treated with the specified drugs at corresponding doses. The drugs were administered every three days for a total of 6 doses. The data are presented as Mean ± SEM.
Results: The dose-dependent tumor growth inhibition (TGI) of OPDIVO® varied; the most significant tumor inhibitory effect was observed in the 10 mpk Group (TGI = 95.0 %); the 0.5 mpk, 1 mpk, and 5 mpk groups displayed comparable tumor volumes, with TGI values of 73.6 %, 66.6 %, and 67.5 %, respectively (Left Figure). The five groups of mice showed identical body weight changes (Right Figure).
The results demonstrated that the B6-hPD1 mice mouse is an ideal animal model for evaluating the efficacy of human PD1 antibodies. GemPharmatech's B6-hPD1 responded to both KEYTRUDA® and OPDIVO® (FDA approved).
4. BALB/c-hPD1/hPDL1: KEYTRUDA® Drug Efficacy Test
The tumor inhibitory efficacy of KEYTRUDA® (Pembrolizumab) was tested in BALB/c-hPD1/hPDL1 mouse models subcutaneously transplanted with CT26-hPDL1(Tg)-mPDL1(KO) tumor cell line.
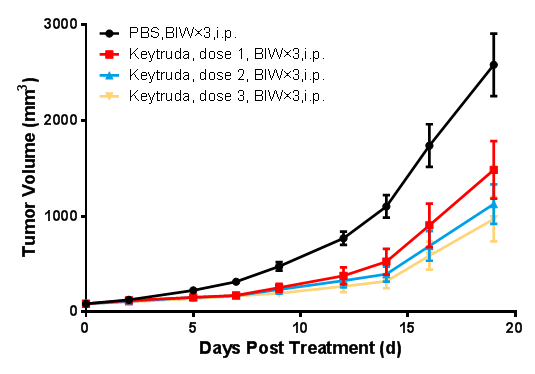
Mouse tumor growth curve.
In the logarithmic growth phase, murine colon cancer cells CT26-hPDL1(Tg)-mPDL1(KO) were subcutaneously inoculated into 6-8 week-old BALB/c-hPD1/hPDL1 mice. When tumors reached an average volume of about 100 mm3, the mice were randomized to PBS Group, Keytruda 0.3 mpk, Keytruda 1 mpk, Keytruda 3 mpk Groups (n = 8) and treated with corresponding drugs administered twice weekly for a total of 6 doses. The data are presented as Mean ± SEM.
Results: Tumor growth inhibition (TGI) was 43.49%, 57.74%, and 65.67%, respectively, in Keytruda 0.3 mpk, Keytruda 1 mpk, and Keytruda 3 mpk Groups. The tested antibodies all showed significant tumor growth inhibition, somewhat dose gradient-dependent.
The results demonstrated that BALB/c-hPD1/hPDL1 mice can be used to evaluate the antitumor effect of anti-human PD1 antibodies, anti-human PDL1 antibodies, and their combination therapies.
5. C57BL/6-hPD1/hPDL1: KEYTRUDA® and TECENTRIQ® Efficacy Test
The tumor inhibitory efficacy of anti-human PD1 antibody KEYTRUDA® (Pembrolizumab) and anti-human PD-L1 antibody TECENTRIQ® (Atezolizumab) was tested in B6-hPD1/hPDL1 mice that was subcutaneously inoculated with MC38-hPDL1(Tg)-mPDL1(KO) tumor cell line.
KEYTRUDA® (Pembrolizumab): an anti-human PD1 antibody antitumor drug marketed by Merck Sharp & Dohme.
TECENTRIQ® (Atezolizumab): an anti-human PD-L1 antibody antitumor drug marketed by Roche.
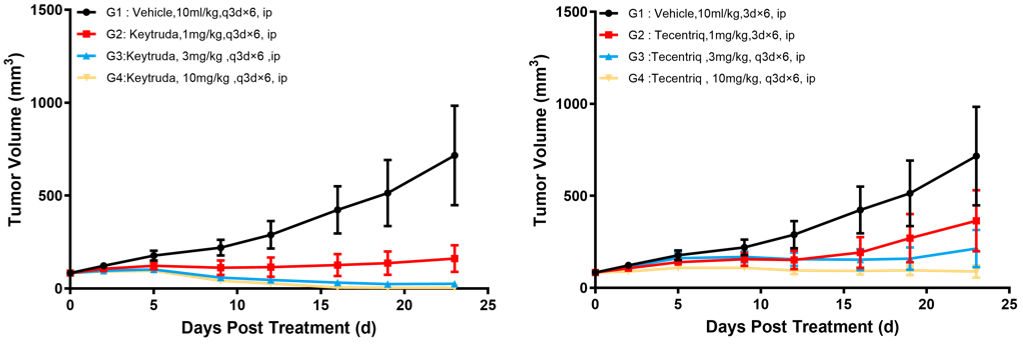
Left Figure: Antitumor efficacy of Keytruda Right Figure: Antitumor efficacy of Tecentriq
In vivo efficacy test in a model of C57BL/6-hPD1/hPDL1 mice subcutaneously inoculated with MC38-hPDL1(Tg)-mPDL1(KO). In the logarithmic growth phase, murine colon cancer cells MC38-hPDL1(Tg)-mPDL1(KO) were subcutaneously inoculated into 6-8 week-old C57BL/6-hPD1/hPDL1 mice. When tumors reached an average volume of about 100 mm3, the mice were randomized to Keytruda 1 mpk, Keytruda 3 mpk, Keytruda 10 mpk, Tecentriq 1 mpk, Tecentriq 3 mpk and Tecentriq 10 mpk Groups (n = 8) and treated with the corresponding drugs. The drugs were administered every three days for a total of 6 doses. The data are presented as Mean ± SEM.
Results: TGI was 79.63%, 96.45% and 99.52%, respectively, in Keytruda 1 mpk, Keytruda 3 mpk and Keytruda 10 mpk Groups; TGI was 48.26%, 67.77%, 88.52%, respectively, in Tecentriq (1 mpk), Tecentriq (3 mpk), and Tecentriq (10 mpk) Dose Groups. Both antibodies significantly inhibited tumor growth in a somewhat dose gradient-dependent way.
These results demonstrated that theC57BL/6-hPD1/hPDL1 mice can be used to evaluate the antitumor effect of anti-human PD1 antibodies, anti-human PDL1 antibodies, and their combination therapies.
References:
[1] PD1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018, 26;379(4):341-351.
[2] PD1 makes waves in anticancer immunotherapy. Nat Rev Drug Discov. 2012, 11(8):601.
[3] Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011,117(17):4501-10.

