Featured on the Cover of Cell Metabolism! Publication on Cancer Cachexia Supported by GemPharmatech’s Mouse Models

GemPharmatech’s mouse models were recently cited in a December publication titled, "Disrupted methionine cycle triggers muscle atrophy in cancer cachexia through epigenetic regulation of REDD1," which was featured on the cover of Cell Metabolism. Read below to learn about this disease and how our models were used in the research.

Figure 1: Cover Image of Cell Metabolism (Image courtesy of the research team)
Cancer cachexia, also known as “wasting syndrome,” is a common and serious complication in cancer patients. It is characterized by weight loss, reduced appetite, skeletal muscle atrophy, and fat degradation, significantly affecting the quality of life and increasing the risk of death. Currently, there are no effective treatments, and traditional nutritional support only partially alleviates symptoms. Therefore, in-depth research into its pathogenesis is especially important.
Methionine is an essential amino acid involved in protein synthesis and cellular epigenetic modifications. S-adenosylmethionine (SAM), the metabolic product of methionine, plays a key role in the regulation of gene expression. The AKT signaling pathway is crucial in regulating cell growth and energy metabolism, but its activation is suppressed during the skeletal muscle degradation process in cachexia. DNA damage-inducible transcript 4 (Ddit4, also known as REDD1), a key inhibitory regulator of AKT pathway activation, is significantly elevated in cachectic skeletal muscle tissue. Inhibition of REDD1 can significantly improve cancer cachexia-related skeletal muscle atrophy. However, the mechanism behind the increased expression of REDD1 in cancer cachectic skeletal muscle remains unclear.
"Disrupted methionine cycle triggers muscle atrophy in cancer cachexia through epigenetic regulation of REDD1" was published online on December 26, 2024, in Cell Metabolism. The work was a collaboration between a research group led by Professor Hongwei Wang and Assistant Professor Zhiqiang Huang of the Medical School of Nanjing University, and a research group led by Professor Yongxiang Wang of Northern Jiangsu People’s Hospital. This study reported for the first time the important metabolic network of skeletal muscle atrophy caused by cachexia, revealing the epigenetic regulatory mechanism of DNMT3A - ATF4 - REDD1, and proposed that restoration of the methionine cycle and SAM supplementation are potential nutritional intervention approaches to ameliorate skeletal muscle atrophy in cachexia.

Figure 2: Research Illustration
The study was initiated by investigating the skeletal muscle phenotype of advanced cancer patients afflicted with cachexia. Using a comprehensive multi-omics strategy integrating metabolomics, genomics and epigenomics, the researchers investigated the pathological features associated with aberrant methionine metabolism during cachexia-induced skeletal muscle atrophy. Both clinical samples and mouse models of cachexia revealed significant disruptions in the methionine cycle, notably an impaired conversion of methionine to S-adenosylmethionine (SAM), leading to a marked reduction of SAM levels in skeletal muscle tissue. The resulting SAM deficiency caused the downregulation of DNMT3A expression, which in turn decreased DNA methylation levels in myofibers. Furthermore, endoplasmic reticulum stress (ERS) was induced, activating the transcription factor ATF4, which increased the expression of the AKT pathway inhibitor, REDD1, in cells. Using transgenic and gene knockout mouse models, the researchers demonstrated that the deletion of REDD1 significantly mitigated tumor-induced weight loss and skeletal muscle degradation, uncovering the epigenetic regulatory mechanism linking methionine metabolism, DNMT3A, ATF4, and REDD1.
The researchers delved into skeletal muscle atrophy in various cachexia animal models, including different modeling approaches (spontaneous and induced), sexes (male and female), and age groups (young and aged). Their findings confirmed the conservation of the epigenetic regulatory mechanism involving DNMT3A-ATF4-REDD1. Moreover, supplementation with methionine and its metabolite, SAM, alleviated cachexia-induced skeletal muscle atrophy, underscoring the crucial role of methionine metabolism in muscle function. Maintaining proper methionine metabolism in skeletal muscle enhanced endoplasmic reticulum function, improved mitochondrial metabolic activity, inhibited autophagy, and suppressed the activation of the ubiquitin-proteasome system, collectively preventing muscle degradation.
From a nutritional standpoint, the research team explored the protective effects of various methyl donors on skeletal muscle atrophy associated with cachexia. Although the folate and methionine cycles are tightly interconnected one-carbon metabolic networks in the body, supplementation with folic acid and betaine did not alleviate tumor-induced skeletal muscle atrophy. In contrast, supplementing with methionine and SAM significantly enhanced the metabolic status of skeletal muscle and mitigated cachexia-induced muscle wasting.
These findings highlight the close relationship between methionine metabolism and cachexia-induced skeletal muscle atrophy, offering potential strategies for nutritional interventions and providing new insights for the clinical management of cancer cachexia. The study identified critical metabolic pathways involved in this process, revealed the epigenetic regulatory mechanisms of DNMT3A/REDD1, and suggested that methionine and SAM supplementation could serve as a promising nutritional approach to counteract cachexia-related muscle atrophy (Figure 3).
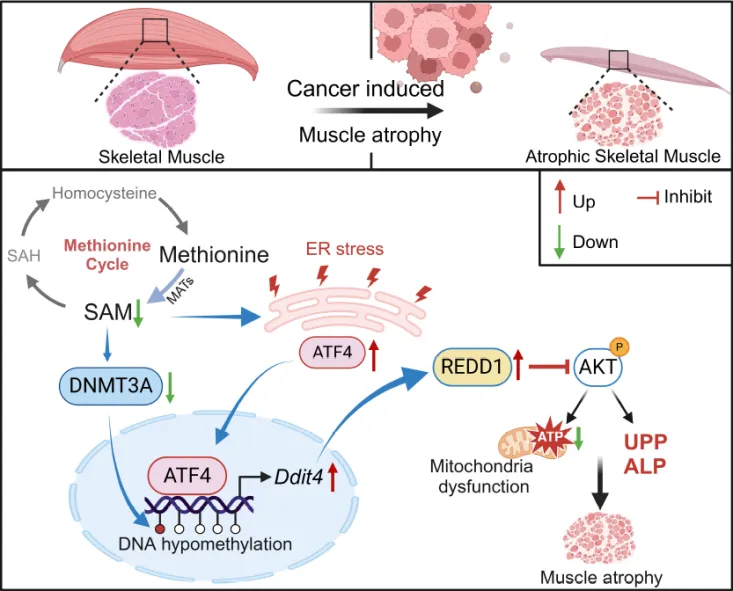
Figure 3: Methionine Cycle-Mediated Skeletal Muscle Metabolic Regulatory Mechanism
GemPharmatech’s mouse models used in this study
Strain Number | Strain Name |
For more information on these models, contact us at:


