Introduction to TCE Drugs
T-cell engagers (TCEs) are innovative bispecific antibodies designed to bind simultaneously to tumor-associated antigens (TAAs) and the CD3 receptor on T-cells. Several TCE drugs have already been approved, with seven bispecific antibodies targeting indications such as multiple myeloma and diffuse large B-cell lymphoma (DLBCL), focusing on markers like BCMA, GPRC5D, CD20, and CD19. In the realm of solid tumors, two TCE bispecific antibodies have received approval: Tebentafusp (Immunocore, January 2022, targeting CD3×GP100) and Tarlatamab (Amgen, May 2024, targeting CD3×DLL3). The accelerated approval of Tarlatamab has spurred increased interest in TCE bispecific antibodies for drug development in the solid tumor space. However, significant unmet clinical needs remain, especially for TCE bispecific antibodies targeting solid tumors.
To address this gap, GemPharmatech has established a comprehensive preclinical pharmacology and efficacy evaluation platform, offering a range of in vivo tumor models to support the development of TCE-based therapies.
In Vivo Antitumor Evaluation Models for TCEs - CD3 Humanized Tumor Models
At the molecular level, TCEs bridge the interaction between tumor cells and T-cells via two primary binding regions: one recognizing T-cell markers (usually CD3) and the other targeting TAAs. This interaction forms an immune synapse that activates T-cells to eliminate tumor cells. Many therapeutic antibodies targeting human CD3 have limited cross-species reactivity with mouse CD3. To overcome this limitation, GemPharmatech has developed the BALB/c-hCD3EDG(Tg)-mCD3EDG(KO) mouse model (BALB/c-hCD3EDG, T053483) based on BALB/c mice.
To demonstrate T cells can be activated through human CD3, splenocytes were isolated from wild type BALB/c or BALB/c-hCD3EDG mice,and stimulated with anti-mCD3 or anti-hCD3 antibodies. As shown in Figure 1, T cells from wild type BALB/c can only be activated by anti-mCD3 but not anti-hCD3, whereas T cells from BALB/c-hCD3EDG mice can only be activated by anti-hCD3 but not anti-mCD3.
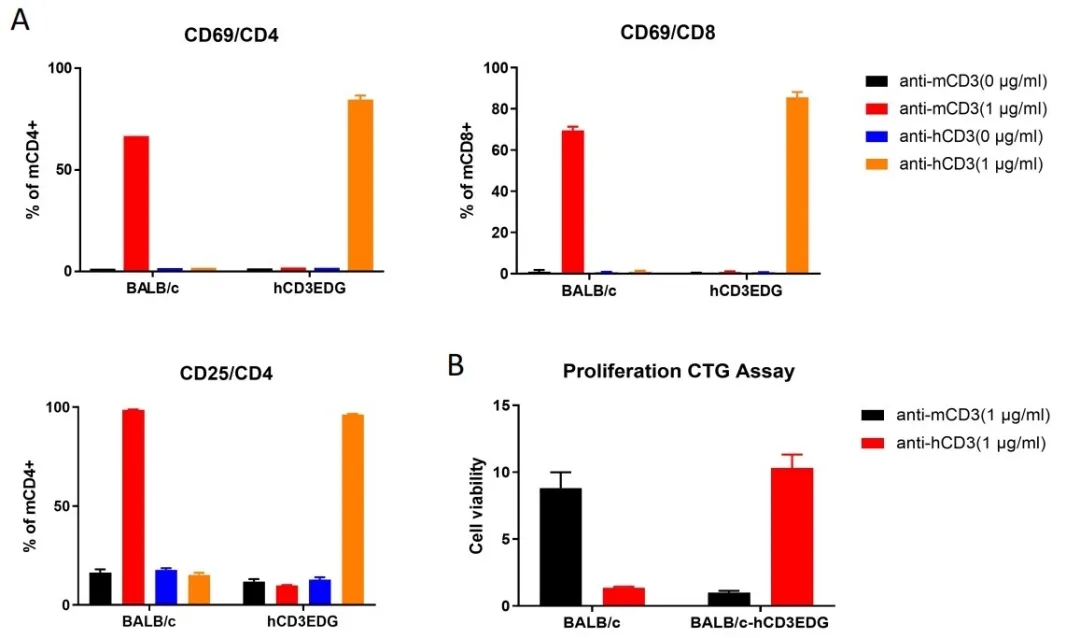
Figure 1: In vitro stimulation of T-cell activation and proliferation by CD3 antibodies.
To evaluate bispecific antibodies such as CD3×CD20, a humanized B cell lymphoma line (A20-hCD20) is implanted subcutaneously into BALB/c-hCD3EDG mice. Significant tumor growth inhibition was observed in the mice treated with CD3/CD20 TCE, as shown in Figure 2.
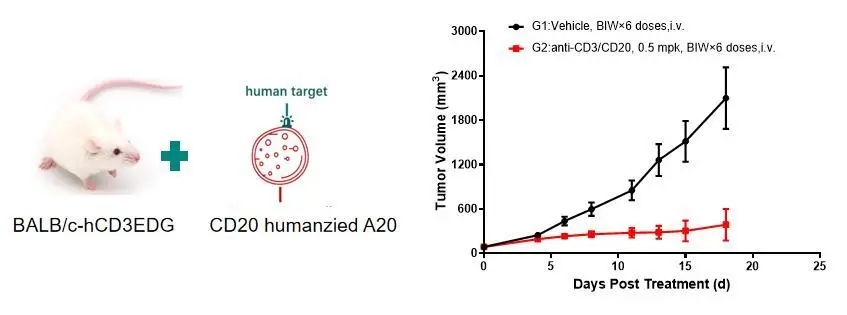
Figure 2: In vivo efficacy evaluation of TCE using the subcutaneous A20-hCD20 cell model in BALB/c-hCD3EDG mice.
BALB/c-hCD3EDG humanized mouse model offers an optimal platform for evaluating the efficacy of human CD3-targeting TCE bispecific antibodies. This makes the BALB/c-hCD3EDG model a crucial tool in advancing the development of novel TCE therapies.
GemPharmatech has developed more than 160 tumor-targeted humanized cell lines and several CD3-humanized mouse models targeting various antigens. By pairing these CD3-humanized mice with the corresponding humanized tumor cell lines, we have created a robust platform for the evaluation of T-cell directed bispecific antibody therapies.
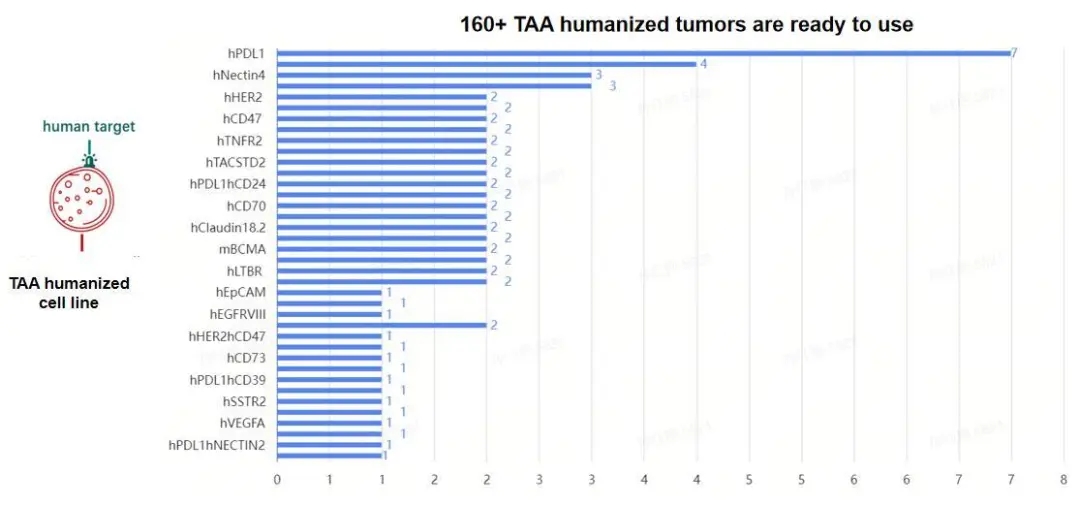
Figure 3: TAA humanized cell line resources.
Table: CD3-TAA humanized mouse model resources
Target | Strain Number | Strain Name | Availability |
CD3E/MSLN | available | ||
CD3E/GPA33 | available | ||
CD3EDG/CD5 | available | ||
CD3EDG/CD22 | available | ||
CD3EDG/CD73 | available | ||
GPRC5D/CD3EDG | available | ||
CD3EGD/EGFR | available | ||
CD3EDG/GPC3 | available | ||
CD3EDG/hBCMA | available | ||
CD3EDG/hGPRC5D | available | ||
CD3EDG/TROP2 | available | ||
CD3EDG/HER2 | available | ||
CD3EDG/CD19 | ongoing | ||
CD3EDG/CEACAM5 | available |


