Immune checkpoint inhibitors targeting PD-1/PD-L1 have revolutionized cancer treatment, offering broad-spectrum efficacy, prolonged durability, and relatively low toxicity[1]. Despite these advancements, the response rates in many cancers remain disappointing, often below 30%[2]. A significant proportion of patients experience partial responses or worse — eventually developing resistance leading to disease progression. These challenges highlight the urgent need for developing novel therapies to overcome anti-PD-1 resistance. This blog will explore how GemPharmatech has developed its own models of anti-PD-1 resistance.
Anti-PD-1 resistance is driven by complex and diverse mechanisms, including tumor antigen loss, MHC inactivation, abnormalities in the IFN-gamma (IFNγ) signaling pathway, and changes in the tumor microenvironment. Understanding these mechanisms is essential to improve existing treatments and develop novel strategies to overcome resistance.
Non-clinical anti-PD-1 resistance models and evaluation systems, based on resistance mechanisms, serve as critical tools for understanding resistance, optimizing treatment strategies, and advancing drug development. Common non-clinical strategies include drug-induced acquired resistance models, gene-editing-based resistance models targeting known genes responsible for drug resistance, and primary resistance evaluation systems. These models cater to different resistance mechanisms and should be selected based on drug mechanism of action and the target clinical population.
Acquired Resistance Models: Mimicking the Evolution of Resistance During Treatment
Acquired resistance occurs when tumor cells gradually adapt to a drug during treatment. This process typically involves genetic mutations, epigenetic modifications, and reprogramming of signaling pathways under the pressure of drug treatment. Acquired resistance models aim to simulate this dynamic process, allowing researchers to study how resistance evolves over time in response to sustained drug exposure. These models help provide insights into the molecular and cellular changes that drive resistance, ultimately aiding in the development of strategies to overcome resistance in patients undergoing PD-1 targeted therapy.
GemPharmatech successfully developed the CT26-PD1-R resistance model by continuously administering Keytruda (Pembrolizumab) to BALB/c-hPD1 mice bearing CT26 tumor. This model demonstrates a complete loss of responsiveness to Keytruda (Figure 1). Tumor-infiltrating lymphocyte (TIL) analysis reveals a marked reduction in the populations of CD335+ NK cells and MHCII+ antigen-presenting cells in the resistant tumors compared to the CT26-WT control, suggesting a significant suppression of immune function within the tumor microenvironment in the CT26-PD1-R model (Figure 2). To gain deeper insights into the resistance mechanisms, further bioinformatics analysis was conducted comparing CT26-PD1-R and CT26-WT cells. The results indicate a notable upregulation of chemokines, including CCL2, CCL5, and CCL7, in the resistant cells (Figure 3). These findings suggest that the tumor microenvironment in the CT26-PD1-R model may facilitate anti-PD-1 resistance by promoting immune suppression, potentially through altered chemokine signaling. This model offers a valuable tool for further investigation into the complex mechanisms underlying immune evasion and resistance to PD-1-targeted therapies.
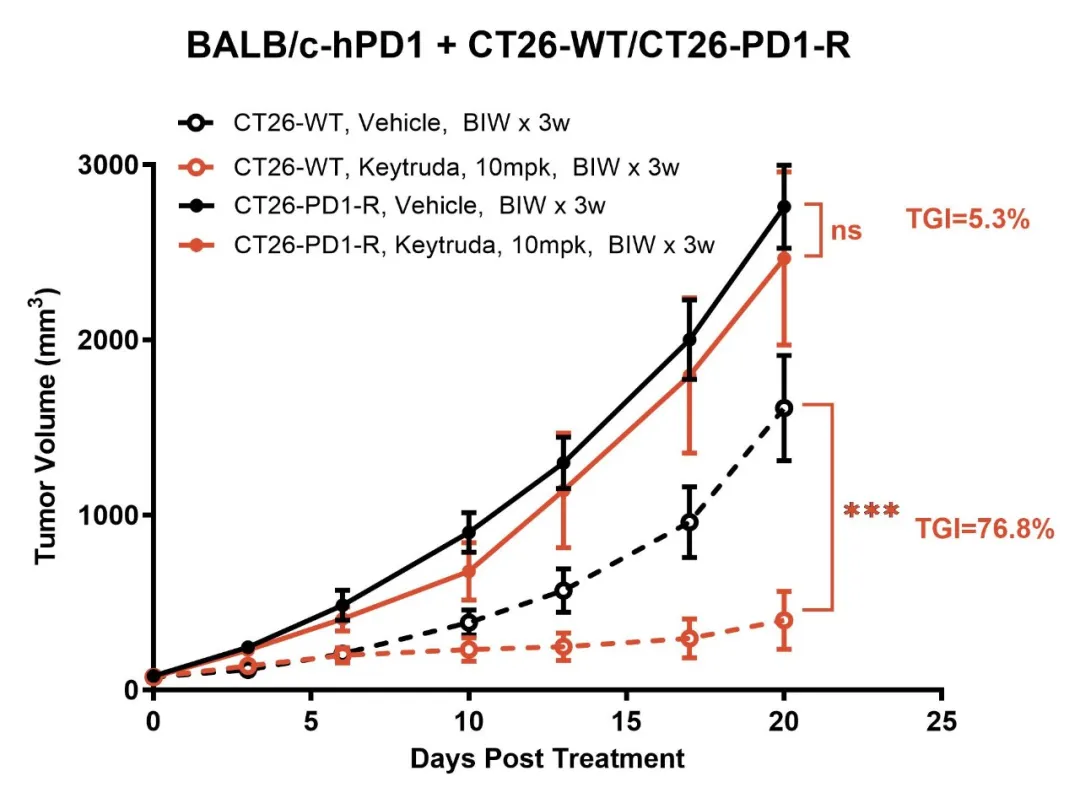
Figure 1: The CT26-PD1-R resistance model shows no response to Keytruda.
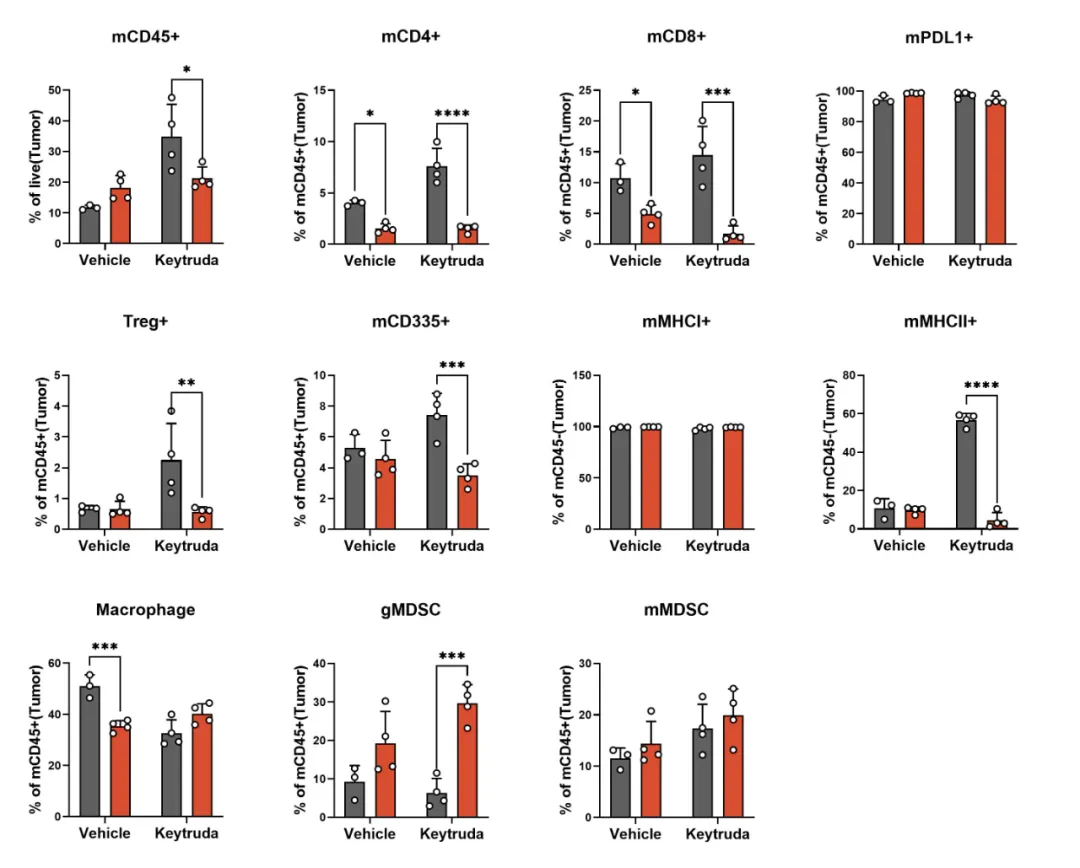
Figure 2: Decreased NK cell infiltration and reduced MHCII expression in the CT26-PD1-R resistance model.
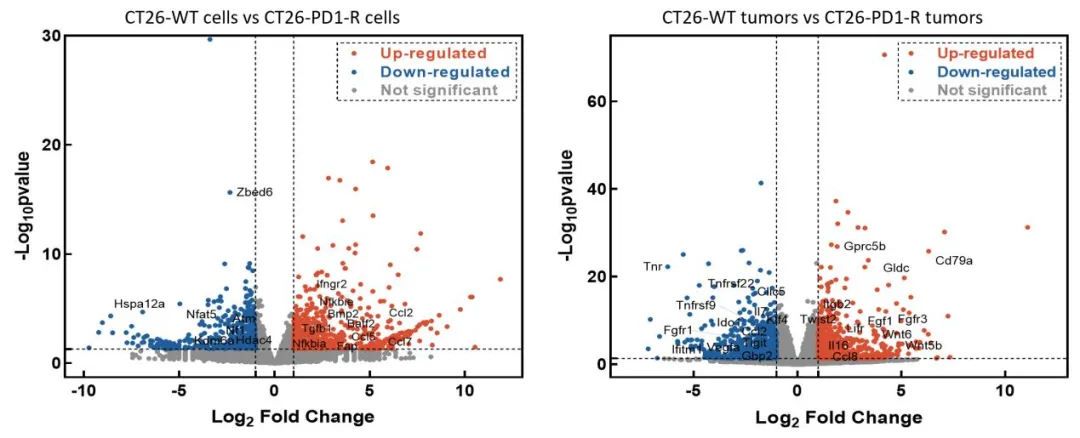
Figure 3: Differential gene expression analysis between CT26-WT and CT26-PD1-R cells.
Gene Editing Models: Targeting Key Genes to Establish Resistance Models
In the investigation of PD-1-targeted immune therapy resistance, mutations in B2M (resulting in MHC I dysfunction) and STK11 have been identified as key factors contributing to resistance observed in clinical patients. GemPharmatech has successfully utilized gene editing technology to generate B2M(KO) and STK11(KO) models, both of which exhibit well-defined resistance mechanisms and provide valuable platforms for evaluating novel therapies targeting these mechanisms.
B2M Knockout Model:
B2M is a vital component of the MHC I molecule, and its loss impairs antigen presentation, preventing T cells from recognizing and eliminating tumor cells. This failure of antigen presentation is a critical mechanism of immune evasion. Using gene editing, we developed the CT26-B2M(KO) model, which demonstrates significant resistance to Keytruda treatment (Figure 4). TIL (tumor-infiltrating lymphocyte) analysis indicates that although T-cell infiltration remains unchanged, MHC I molecules are completely absent in the tumor (Figure 5), confirming antigen presentation deficiency as a key factor in immune escape.
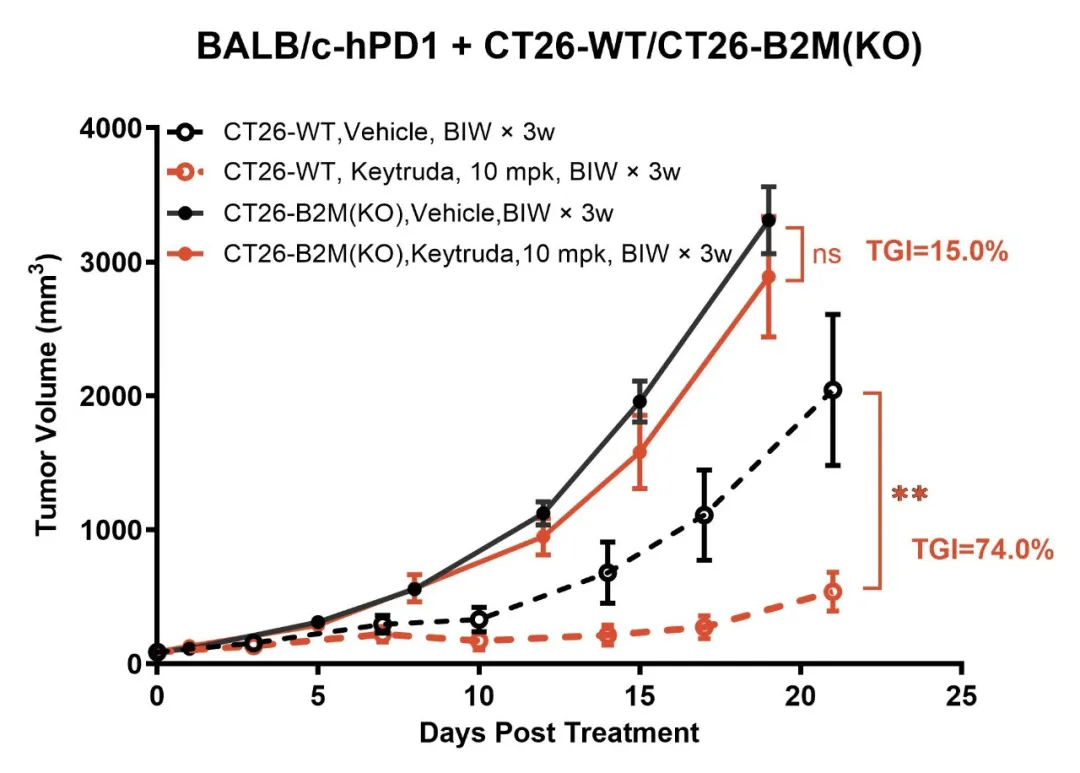
Figure 4: The CT26-B2M(KO) model exhibits resistance to Keytruda treatment.
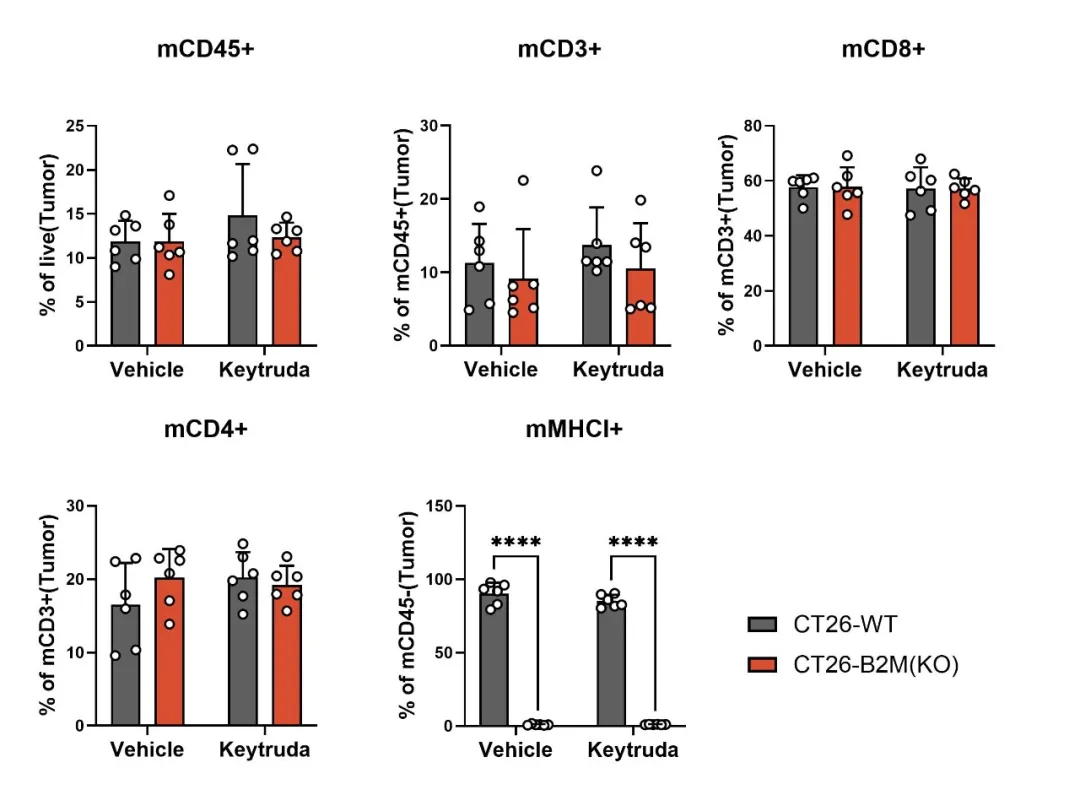
Figure 5: MHC I expression is completely absent in the CT26-B2M(KO) model.
STK11 Knockout Model:
STK11 is a critical tumor suppressor gene, and its loss is strongly linked to metabolic abnormalities and immune suppression within tumors. Using gene editing technology, we created the CT26-STK11(KO) model, which demonstrates robust resistance to Keytruda (Figure 6). TIL analysis reveals a marked reduction in CD8+ cytotoxic T-cell infiltration and a significant accumulation of granulocytic myeloid-derived suppressor cells (gMDSCs) (Figure 7). Additionally, PD-L1 expression on tumor cells is reduced. These findings suggest that STK11 loss not only leads to a reduction in antigen expression but also enhances immune-suppressive signaling, contributing to the development of resistance to anti-PD-1 therapy.
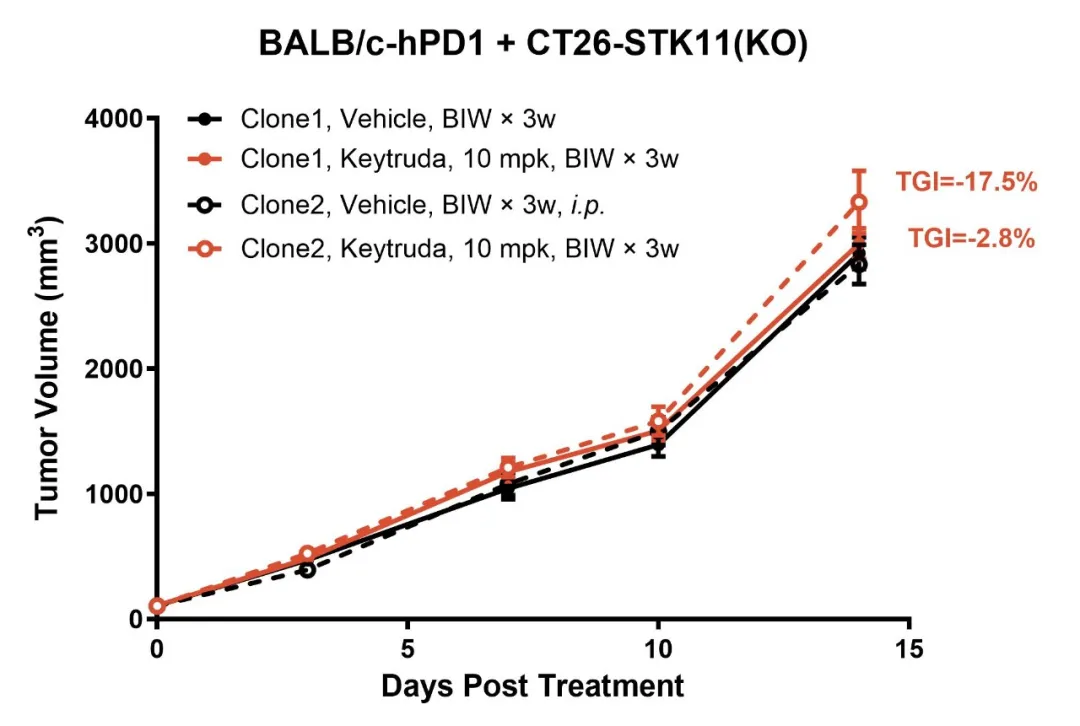
Figure 6: The CT26-STK11(KO) model exhibits resistance to Keytruda treatment.
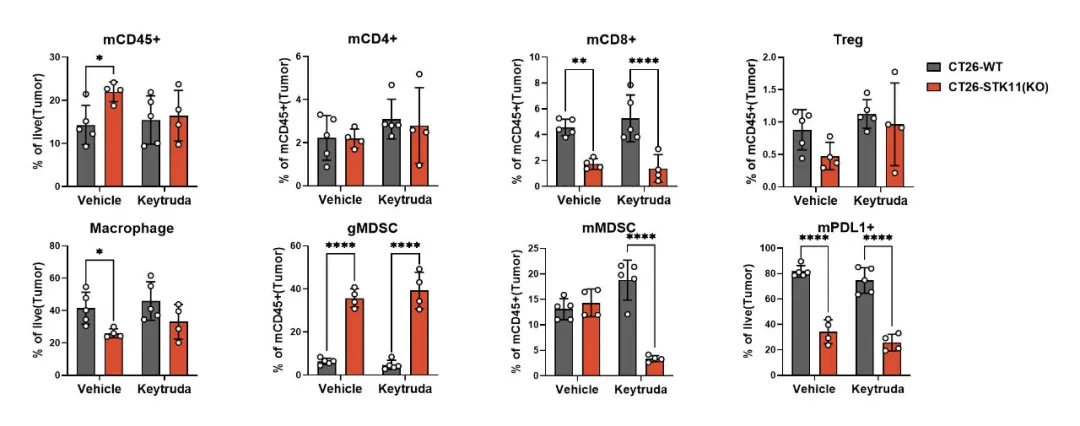
Figure 7: Reduced CD8+ T-cell infiltration and decreased PD-L1 expression are observed in the CT26-STK11(KO) model.
Primary Resistance Models: Identifying Non-Responding Tumor Populations Early in Treatment
Primary resistance refers to the phenomenon where tumors exhibit little to no response to treatment from the outset, even before any therapeutic intervention. This resistance is often attributed to inherent genetic mutations or specific gene expression profiles within the tumor cells. By analyzing primary resistance samples, we can gain deeper insights into the molecular mechanisms underlying resistance, which can, in turn, inform the development of next-generation therapeutics.
GemPharmatech provides a comprehensive set of PD-1 primary resistance models that include a variety of tumor cell lines, such as Panc02, B16F10, LLC1, Renca, MB49, MPC11, and mPAKPC (Figures 8-9). These models facilitate the investigation of resistance mechanisms at an early stage in the treatment process. Notably, mPAKPC cells, derived from KPC mice (Pdx1-cre/LSL-Kras G12D/P53 (R172H)), spontaneously develop pancreatic cancer and exhibit inherent resistance to PD-1 therapy in vivo (Figure 9).
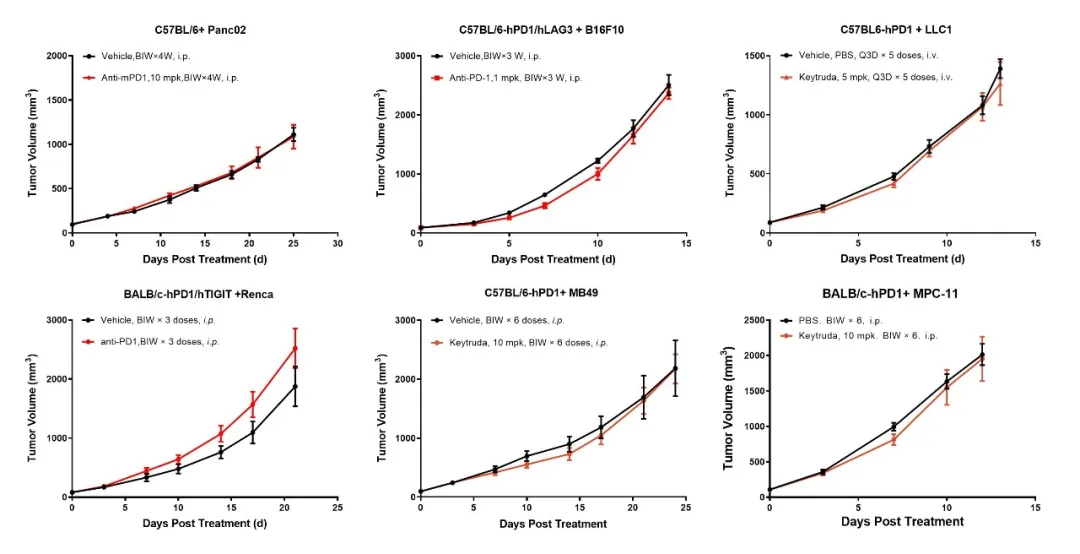
Figure 8: The tumor cell lines Panc02, B16F10, LLC1, Renca, MB49, and MPC11 exhibit no response to Keytruda treatment.
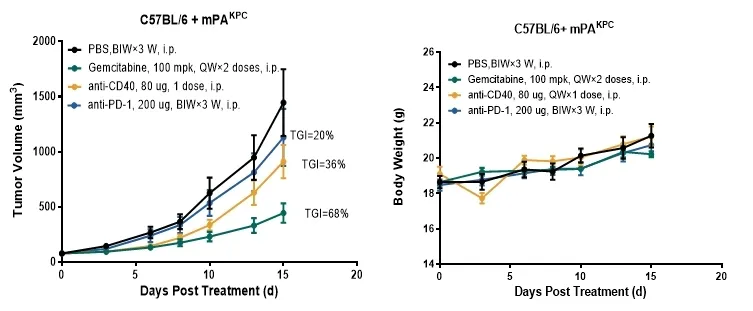
Figure 9: The mPAKPC pancreatic cancer model demonstrates resistance to Keytruda treatment.
Conclusion
In summary, for the non-clinical evaluation of novel therapeutics targeting anti-PD-1 resistance, it is recommended to select the appropriate resistance model(s) based on the drug's mechanism of action (MOA) . These models can be effectively applied to pharmacodynamic and pharmacokinetic studies as well as IND applications. GemPharmatech, with its profound expertise in the development and application of anti-PD-1 resistance models, offers robust platforms that are adaptable to various drug evaluation needs.
Work with GemPharmatech
With a comprehensive non-clinical evaluation platform, backed by multi-dimensional data analysis including TIL analysis, immune microenvironment profiling, and genomic and transcriptomic studies, GemPharmatech provides a full range of solutions for optimizing anti-PD-1 therapies.
Explore the services we offer that play a pivotal role in developing innovative treatments for resistant patient populations, or contact us directly at:
Reference:
1. Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021 Oct 14;184(21):5309-5337.
2. Gauci ML, Lanoy E, Champiat S, Caramella C, Ammari S, Aspeslagh S, Varga A, Baldini C, Bahleda R, Gazzah A, Michot JM, Postel-Vinay S, Angevin E, Ribrag V, Hollebecque A, Soria JC, Robert C, Massard C, Marabelle A. Long-Term Survival in Patients Responding to Anti-PD-1/PD-L1 Therapy and Disease Outcome upon Treatment Discontinuation. Clin Cancer Res. 2019 Feb 1;25(3):946-956.


