Hypertensive heart failure (HHF) develops due to chronic pressure overload, leading to left ventricular hypertrophy (LVH), diastolic dysfunction, and eventual systolic impairment. The transverse aortic constriction (TAC) model in mice mimics this pathophysiology by mechanically constricting the aorta, inducing pressure overload, which results in left ventricle hypertrophy, myocardial fibrosis, diastolic dysfunction and progression to systolic dysfunction. TAC enables study of hypertrophy-to-failure transition and is useful for testing antihypertrophic therapies (e.g., ARBs, neprilysin inhibitors).
The transverse aortic constriction (TAC) model serves as a robust and clinically relevant tool for studying hypertensive heart failure (HHF). By inducing pressure overload, TAC effectively recapitulates key features of human HHF, including left ventricular hypertrophy, diastolic dysfunction, and eventual systolic impairment. The non-ventilated modification further enhances translational utility by minimizing surgical trauma while preserving physiological respiration. Despite its technical demands, TAC offers unparalleled insights into disease progression and therapeutic interventions, making it indispensable for preclinical HHF research. TAC mimics human hypertensive heart disease but requires rigorous surgical standardization.
Model Validation and Example Data
Doppler analysis of aortic blood flow velocity
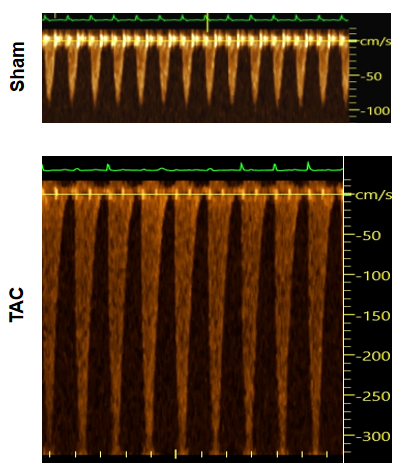
Figure 1. Pulse wave doppler results showing aortic blood flow in the TAC model. Compared to sham samples, TAC mice show dramatically increased flow velocity, which demonstrates alternation of blood dynamics after surgical induction
Cardiac function evaluation in 28G TAC model
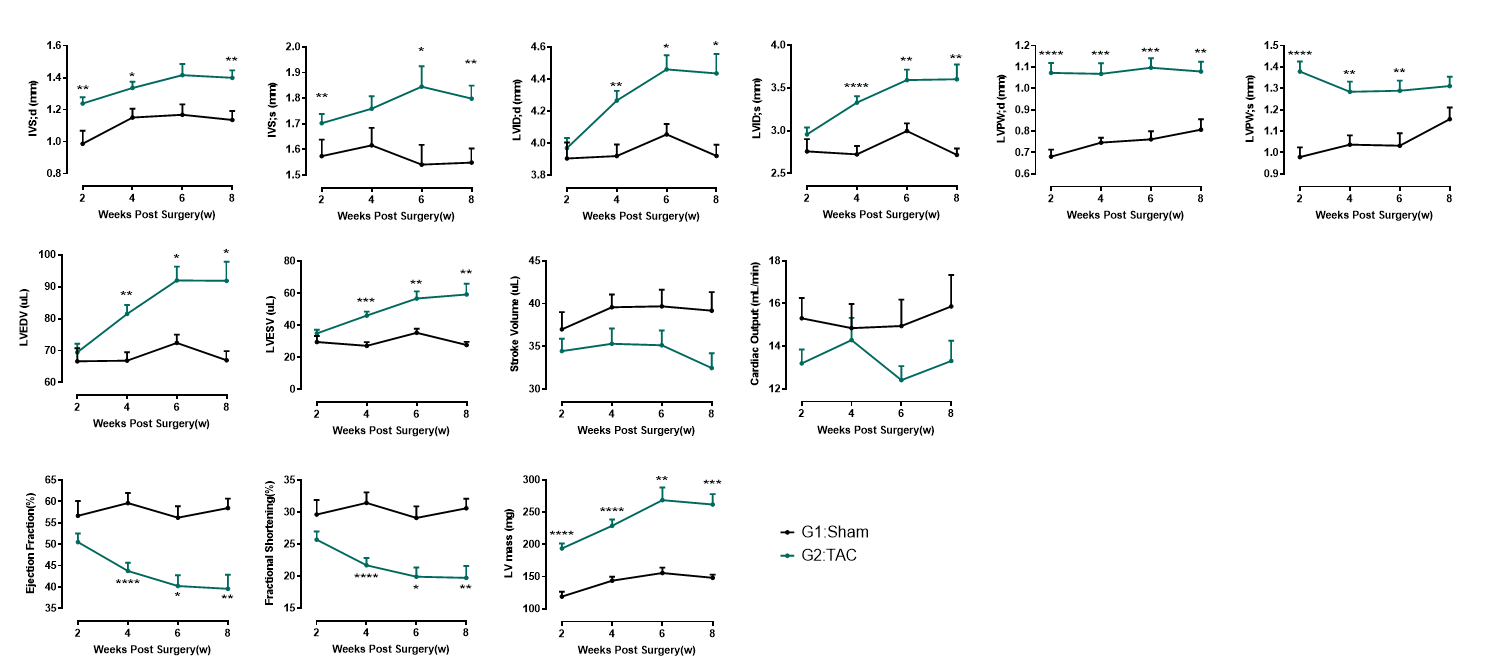
Figure 2. Echocardiography in PSAX (28G). At 2 weeks post-TAC surgery, 28G TAC hearts exhibit a distinct myocardial hypertrophy phenotype, characterized by thickened ventricular walls without significant chamber dilation or systolic dysfunction. This stage represents the compensated pathological hypertrophy phase. By 4 weeks post-surgery, the left ventricular internal diameter (LVID) significantly increases, accompanied by reduced ejection fraction (EF), marking the transition from compensated hypertrophy to decompensation and eventual progression to heart failure.
Cardiac function evaluation in 27G TAC model
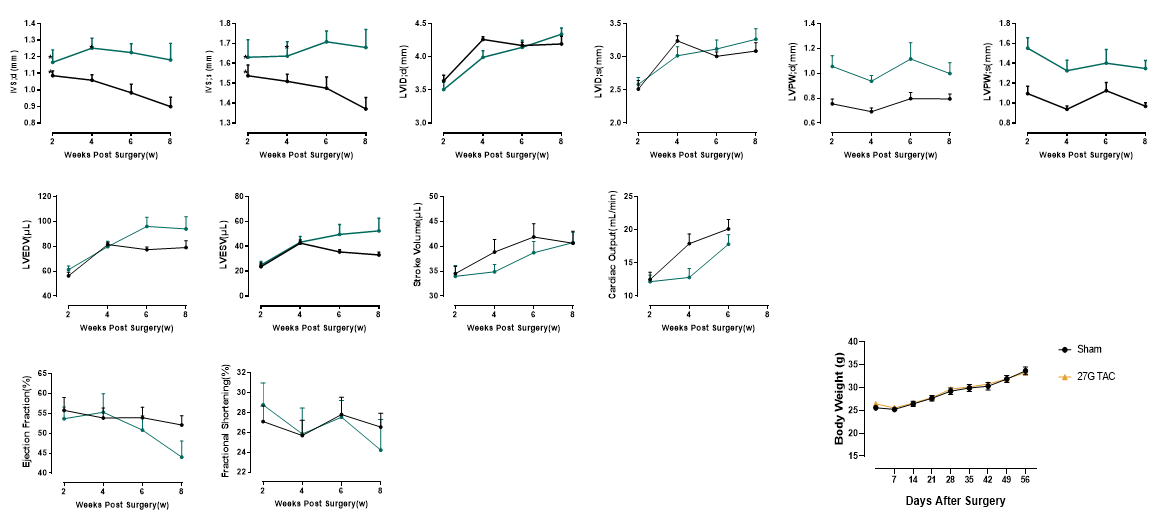
Figure 3. Echocardiography in PSAX (27G). The 27G TAC model induces sustained myocardial hypertrophy for up to 6 weeks post-surgery, without significant ventricular dilation or systolic dysfunction. This model maintains a prolonged compensated hypertrophy phase, making it ideal for studying pathological hypertrophy mechanisms, early-stage cardiac remodeling, and anti-hypertrophic therapies
Cardiac remolding in 28 TAC hearts
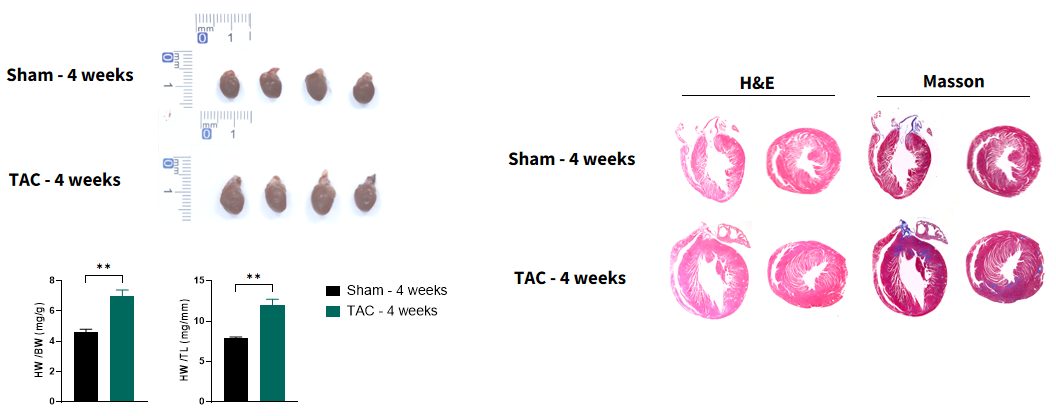
Figure 4. Pathology analysis of TAC model. At 4 weeks post-28G TAC surgery, TAC mice exhibit significant cardiac enlargement and increased heart weight-to-body weight ratio. Pathological results show myocardial hypertrophy and cardiac fibrosis.
Entresto rescues cardiac function and remolding in TAC
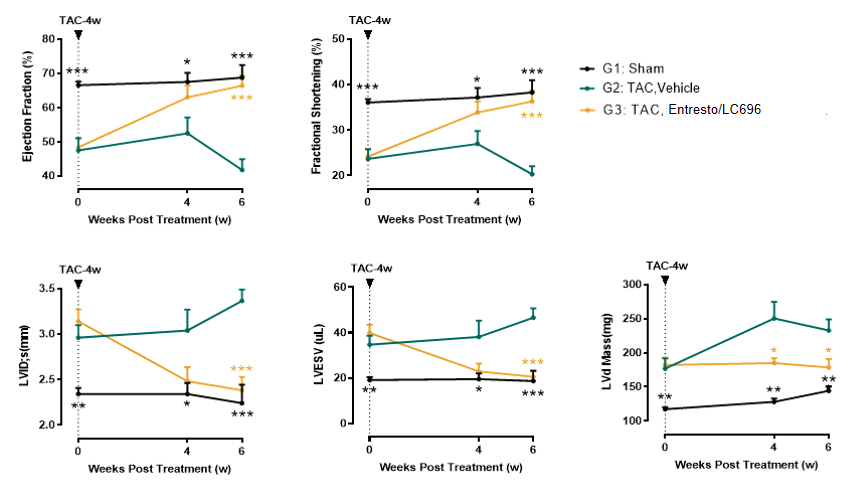
Figure 5. Four weeks post 28G transverse aortic constriction (TAC) surgery, mice exhibit significant cardiac remodeling characterized by ventricular dilation (increased LVID;s and LVESV) and systolic dysfunction (EF reduced to 40-50%). Administration of Entresto (sacubitril/valsartan) at this stage demonstrates remarkable therapeutic effects: echocardiographic assessments at 4- and 6-week post-treatment reveal complete rescue of ventricular dilation and systolic dysfunction (normalization of EF and FS), along with partial rescue of myocardial hypertrophy (reduced LV Mass).