Evaluating CD20 Antibody Efficacy Using BAFF/CD20 Dual-Target Humanized Mice
A novel spontaneous SLE model for testing B-cell–depleting therapies

B-cell–driven autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis are characterized by overactive B-cell signaling, autoantibody production, and chronic inflammation. CD20, a B-cell surface marker critical for activation and differentiation, has emerged as a key therapeutic target in these disorders. Antibodies that deplete B-cells, such as Rituximab, have demonstrated strong clinical efficacy, but preclinical evaluation requires models that faithfully replicate human immune biology.
To advance the evaluation of B-cell–targeted therapies, GemPharmatech scientists developed a dual-humanized spontaneous SLE model that co-expresses human BAFF and CD20. This model was generated by crossing the established B6-hBAFF transgenic strain (T036794), which exhibits spontaneous SLE-like pathology, with CD20-humanized mice (BALB/c-hCD20, T057498) to create BAFF/CD20 dual-target humanized mice (T065937).
Study Design and Findings
The hBAFF/hCD20 mice exhibited autoimmune phenotypes consistent with the B6-hBAFF strain, including elevated serum IgG and IgM beginning at eight weeks of age and increasing with time. High levels of anti-dsDNA autoantibodies, increased CD19+ B-cell ratios, and a rise in plasma B cells confirmed disease progression.
Following treatment with Rituximab, a marked reduction in IgG, IgM, and anti-dsDNA antibody levels was observed. Flow cytometry revealed a significant decrease in circulating CD19+ B cells and plasma cells, indicating effective B-cell depletion.
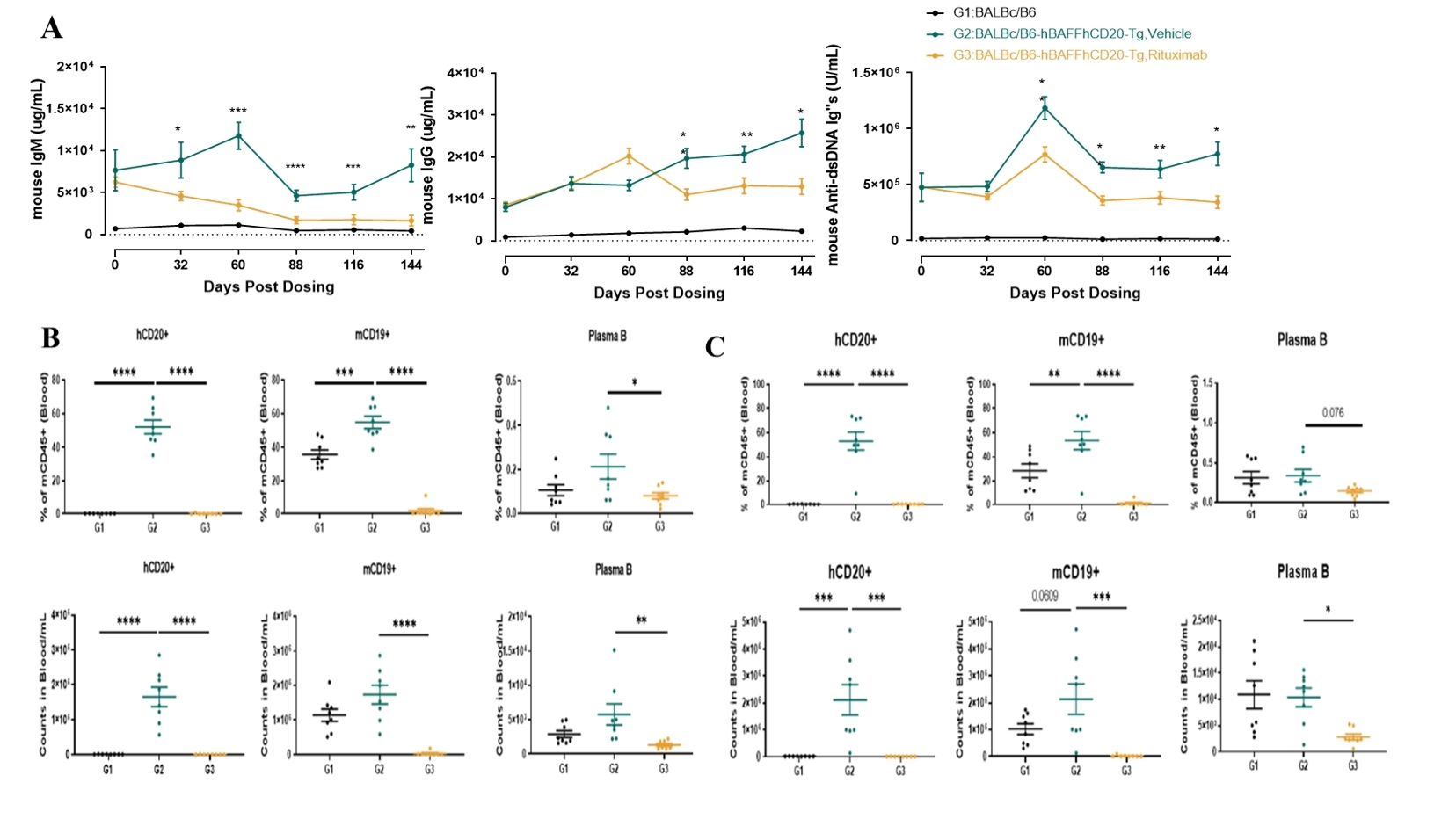
Figure 1. Efficacy evaluation of Rituximab in a spontaneous SLE model based on hBAFF/hCD20 mice. (A) ELISA for serum IgG, IgM, and anti-dsDNA levels from 8 to 28 weeks of age. (B–C) Flow cytometry analysis showing changes in hCD20+ and mCD19+ B-cell and plasma cell populations after 79 and 144 days of treatment with Rituximab.
Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ***p < 0.0001 versus control by one-way ANOVA.
Therapeutic Response
Histological analysis further supported these findings. Untreated hBAFF/hCD20 mice exhibited a high NIH kidney activity score, glomerular basement membrane thickening, and mesangial cell proliferation on HE staining, along with increased glomerular PAS-positive expression. Rituximab administration markedly reduced these pathological signs, confirming therapeutic efficacy.
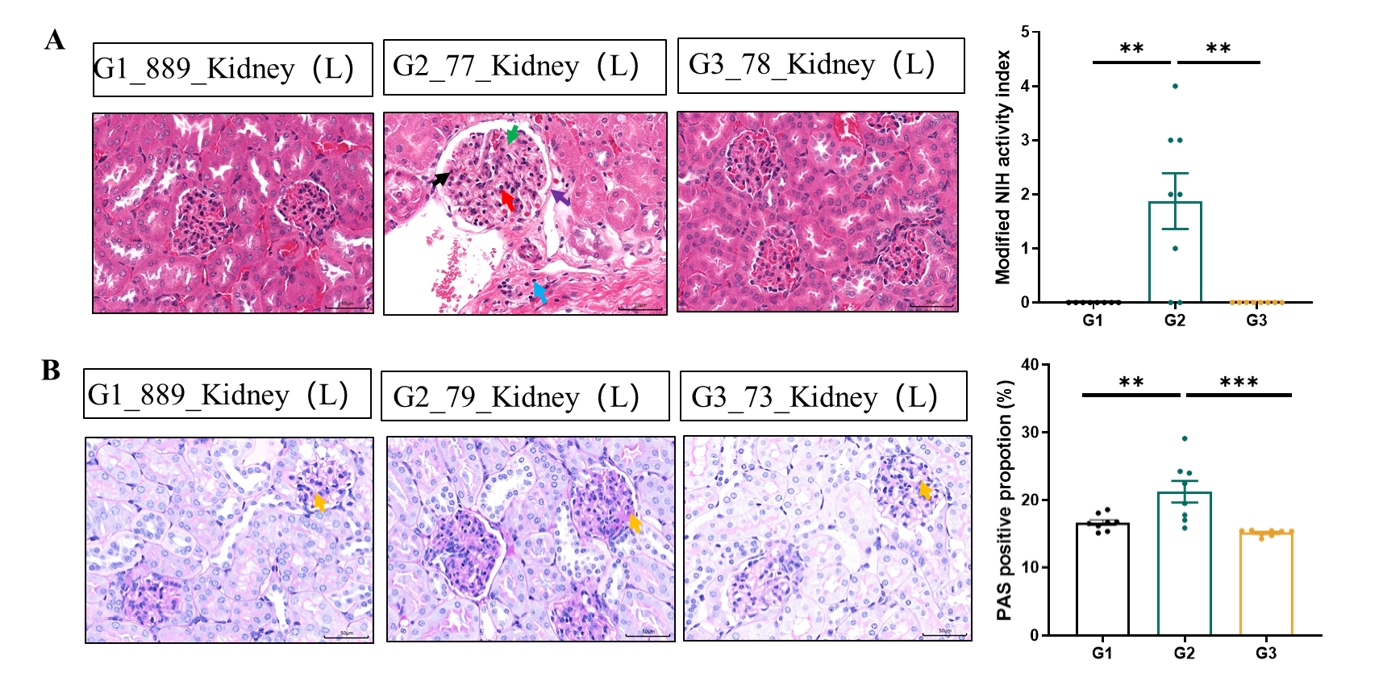
Figure 2. Histopathological staining results of kidney in a spontaneous SLE model based on hBAFF/hCD20 mice. (A) NIH activity score of kidney tissue by HE staining. Arrows indicate glomerular basement membrane thickening (purple), mesangial cell proliferation (red), matrix deposition (green), nuclear fragmentation (black), and lymphocyte infiltration (blue). (B) Quantification of PAS-positive glomeruli showing significant attenuation following Rituximab treatment. Data are presented as mean ± SEM. **p < 0.01; **p < 0.001 versus control by one-way ANOVA.
Expanding Translational Capabilities
The hBAFF/hCD20 dual-target humanized mouse establishes a powerful new platform for evaluating B-cell–depleting therapies and other biologics targeting human CD20 or BAFF pathways. Its spontaneous autoimmune phenotype enables researchers to observe disease onset and therapeutic response without artificial induction, creating a more translatable model for autoimmune research.
Building on this success, GemPharmatech has expanded its portfolio of spontaneous SLE models derived from B6-hBAFF mice, including:
Together, these models support a comprehensive approach to studying the mechanisms of B-cell dysregulation and evaluating novel immunotherapies.
Backed by the world’s largest collection of genetically engineered mouse models, GemPharmatech partners with researchers to accelerate autoimmune drug discovery. Our team combines deep immunology expertise, validated humanized models, and integrated preclinical testing services to help de-risk development and move therapies confidently from discovery to IND.
Click here, or email us at sales@gempharmatech.com to contact us to explore how GemPharmatech’s autoimmune disease models can support your next study!


