Accelerating Anti-Myopathy Drug Discovery: A Dexamethasone-Induced Sarcopenia Model for Preclinical Evaluation

Sarcopenia, the progressive loss of skeletal muscle mass and function, is a major contributor to frailty, falls and chronic morbidity in aging populations. Beyond physical decline, sarcopenia increases susceptibility to cardiovascular, respiratory, and cognitive disorders, significantly impacting the quality of life and mortality.
Despite its growing prevalence, no FDA-approved pharmacological therapy currently exists for sarcopenia. Preclinical animal models are essential for understanding disease mechanisms and evaluating therapeutic candidates, but traditional models such as aged or genetically modified mice require long study timelines and often show high variability.
To help fill this unmet need, GemPharmatech has a suite of reliable translational models for sarcopenia and muscle wasting, including:
Dexamethasone-induced model
CVMD-related cachexia-induced model
MSTN over-expression-induced model
Among these, the dexamethasone-induced model provides a rapid, reproducible, and cost-effective platform for preclinical drug evaluation. A synthetic glucocorticoid, dexamethasone triggers catabolic signaling pathways that lead to muscle wasting, similar to patients with Cushing’s syndrome or those under long-term glucocorticoid therapy.
Unlike aging or genetic models, the dexamethasone-induced sarcopenia requires only one to four weeks to reproduce significant muscle atrophy, which shorten study timelines. This makes the model well-suited for large-scale preliminary screening projects and mechanistic studies.
Functional and Phenotypic Characterization
GemPharmatech established a dexamethasone-induced sarcopenia mouse model to evaluate disease progression and therapeutic rescue. The study assessed functional performance, histopathology, and body composition changes, followed by intervention with Bimagrumab, a monoclonal antibody targeting the activin type II receptor to promote muscle growth.
Body Composition and Exercise Performance Study Design
Dexamethasone treatment resulted in significant reduction of lean body mass and a decline in treadmill running capacity.
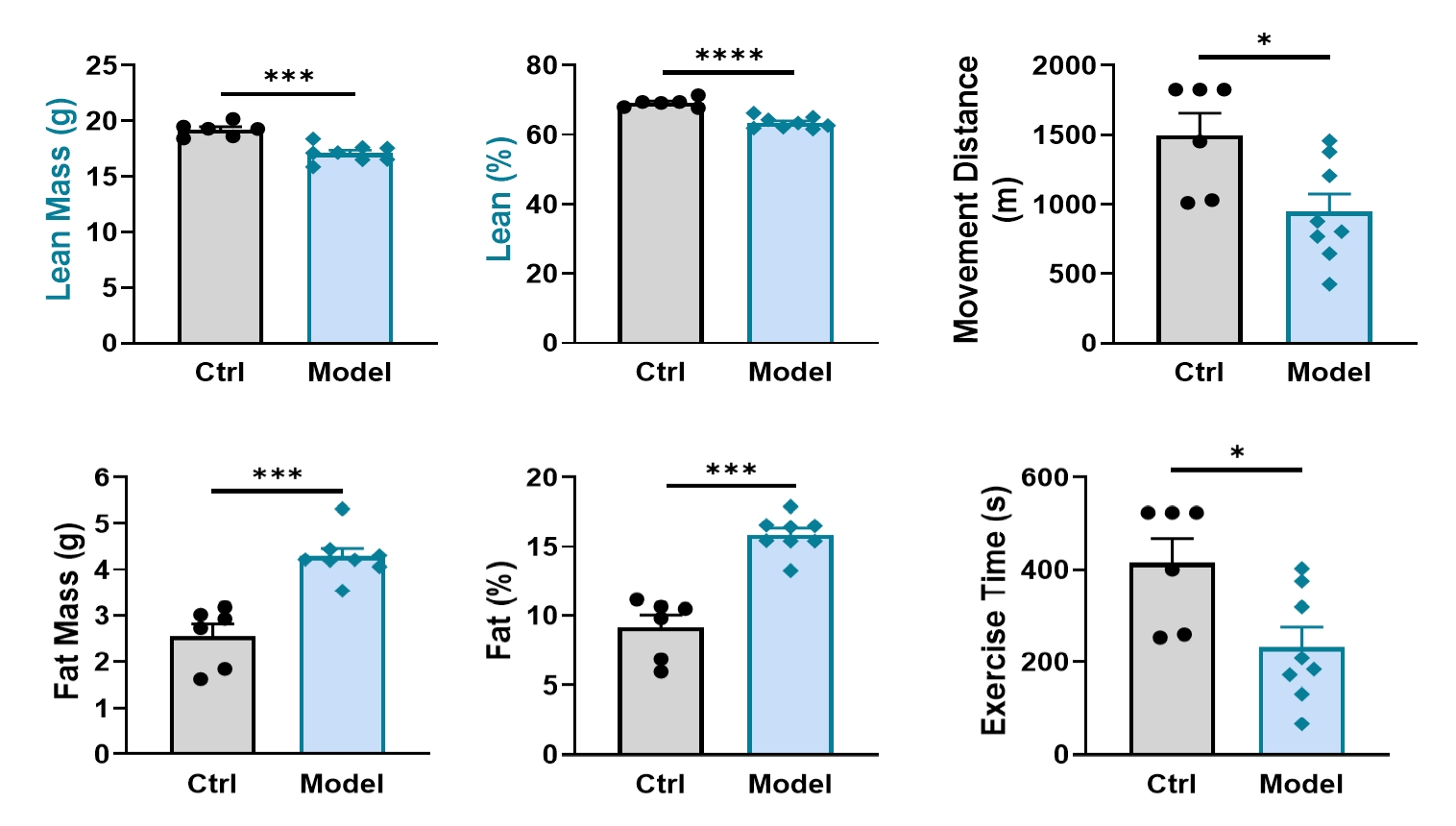
Figure 1: Body composition and treadmill exercise performance following dexamethasone treatment. n=6-8 for each group, data was presented as mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001 versus control group (t-test).
Wheat Germ Agglutinin (WGA) staining showed a significant reduction in muscle fiber cross-sectional area in the gastrocnemius and soleus following dexamethasone induction.
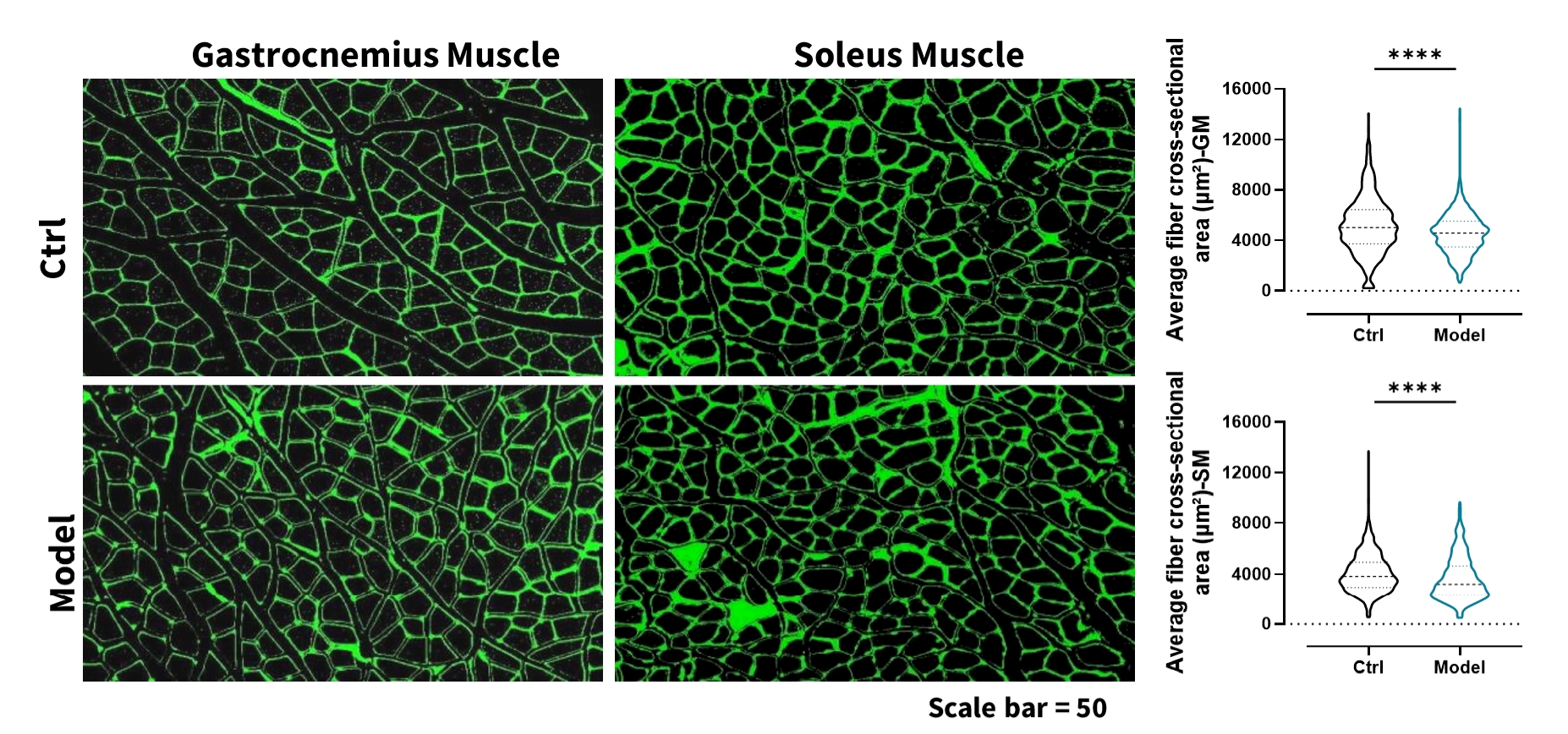
Figure 2: Histopathological analysis of gastrocnemius muscle and soleus muscle. n=6-8 for each group, data was presented as mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001 versus control group (t-test).
Therapeutic Rescue of Muscle Atrophy with Bimagrumab
Following induction of sarcopenia, mice treated with Bimagrumab experienced improved muscle mass and body composition compared to untreated controls.
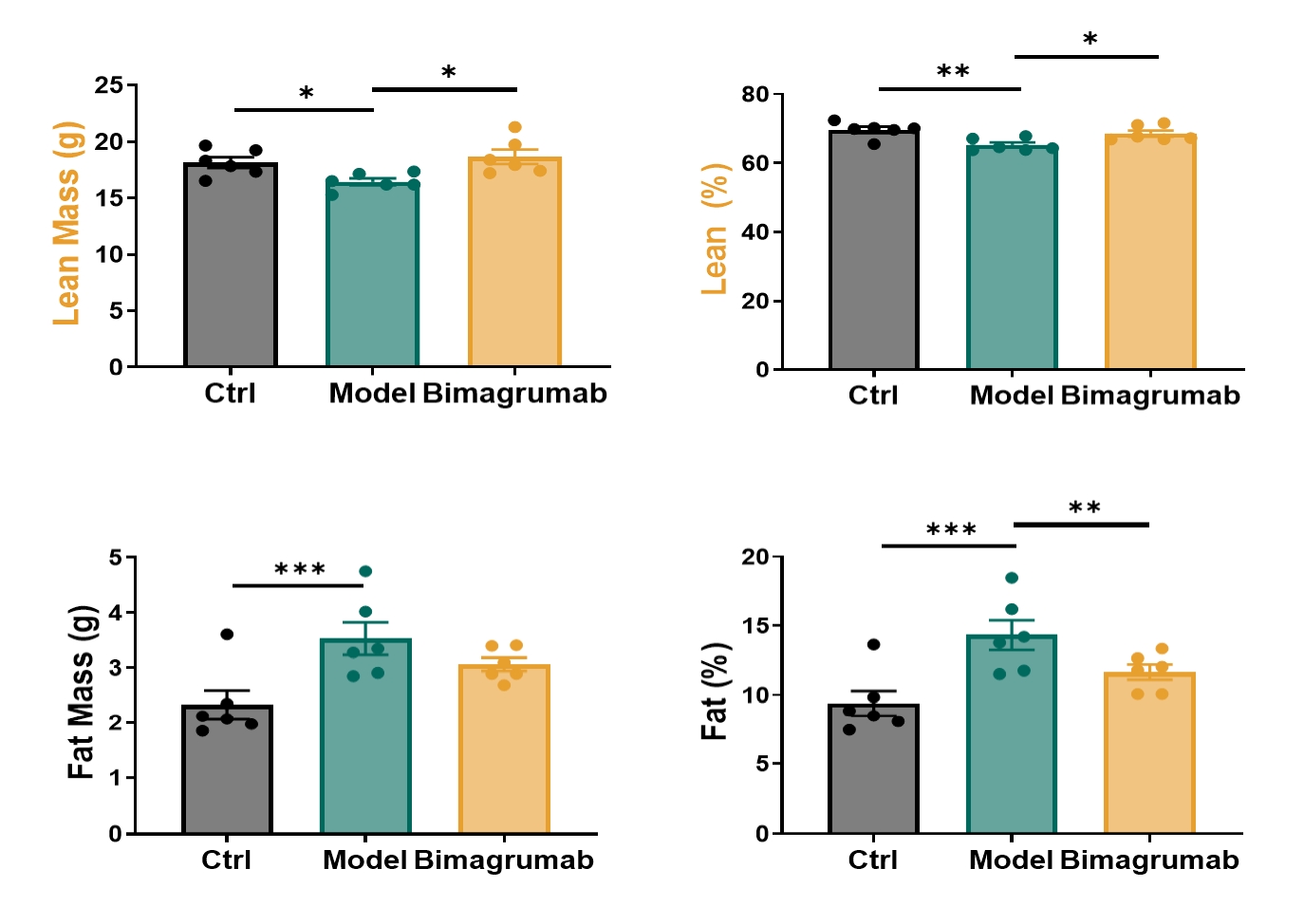
Figure 3: Effect of Bimagrumab on body composition in sarcopenic mice. n=6-7 for each group, data was presented as mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001 versus the model group by ordinary one-way ANOVA.
Bimagrumab-treated mice showed a notable restoration of lean body mass relative to untreated sarcopenic mice. The recovery trend approached baseline levels observed in the control group, suggesting a strong protective effect against glucocorticoid-induced loss.
![]()
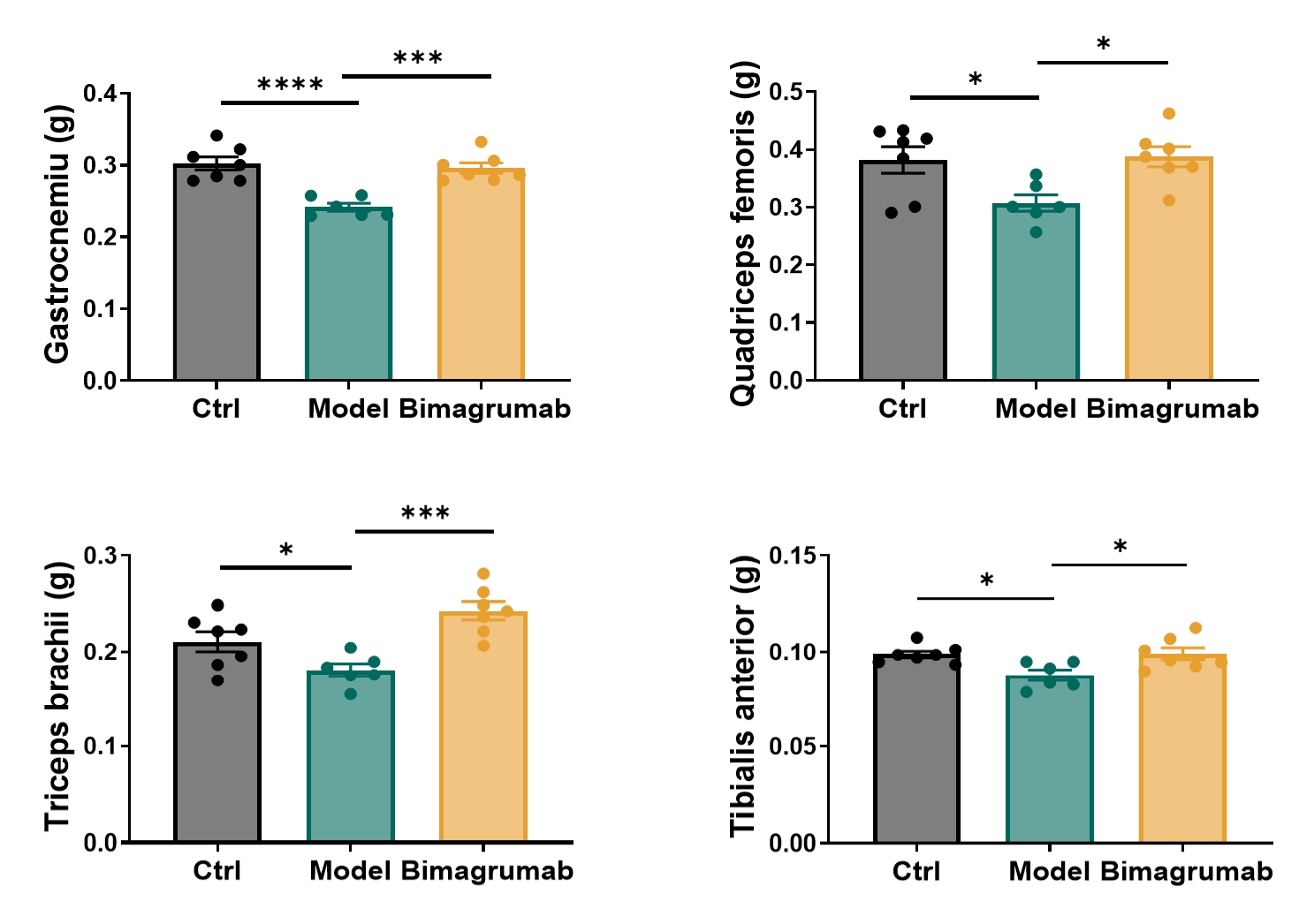
Figure 4: Effect of Bimagrumab on the weight of muscle in sarcopenic mice. n=6-7 for each group, data was presented as mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001 versus the model group by ordinary one-way ANOVA.
Bimagrumab reversed localized muscle loss at the tissue level. Post-treatment analysis indicated increases in gastrocnemius and soleus muscle weights.
Why It Matters
This case study underscores GemPharmatech’s ability to design and execute comprehensive preclinical research studies that integrates:
Validated disease models
Functional assessments, histological analysis
Therapeutic efficacy testing
The dexamethasone-induced sarcopenia model provides a robust platform to investigate mechanisms of muscle loss and to test emerging treatments. By combining deep expertise in model development with translational study design, GemPharmatech empowers drug developers to bridge the gap between discovery and clinical translation, advancing the next generation of anti-myopathy treatments.


