Modeling Hypertrophic Cardiomyopathy: Next-Generation Mouse Models for Translational Research

Hypertrophic Cardiomyopathy (HCM) is the most common inherited heart disease, affecting up to 1 in 200 individuals worldwide [1,2]. Its main characteristics include thickening of the ventricular walls and impaired cardiac function, leading to progressive dyspnea, angina pectoris, heart failure, atrial fibrillation, and sudden cardiac death. While current treatments can alleviate symptoms in some patients, they fail to address the underlying pathophysiological mechanisms of the disease. Novel therapeutic strategies targeting sarcomeric proteins have raised hope, but there remains an urgent need for preclinical models that replicate HCM pathology.
Approximately 55% of HCM cases are associated with genetic mutations occur in genes encoding sarcomeric proteins. Of these, Mybpc3 and Myh7 are the two of the most common, together accounting for nearly 70% of HCM cases caused by genetic mutations [3,4].
MYBPC3 mutations often cause loss of function or insufficient protein expression
MYH7 mutations disrupt sarcomere contractility, frequently though reduced interaction with MYBPC3
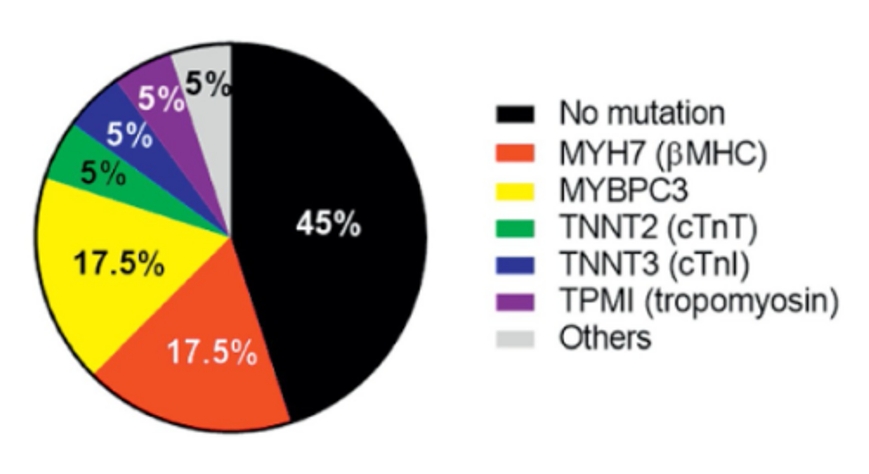
Reference: Trends Mol Med. 2019 Sep;25(9):775-790.
Figure 1: Genetic Causation (17.5% Myh7 mutation and 17.5% Mybpc3 mutation in 55% HCM associated with gene mutations)
To help meet bridge the gap between genetic insights and translational application, GemPharmatech has developed two mouse models that closely replicate human HCM pathogenesis: the Mybpc3 gene knockout (KO) mice and Myh6 R404Q point mutation mice models.
Mybpc3 KO Eccentric Hypertrophic Cardiomyopathy Model
Loss-of-function or insufficient expression of Mybpc3 is common in human HCM. Mybpc3 KO mimics the loss-of-function of human Mybpc3 gene and exhibit characteristics of eccentric hypertrophic cardiomyopathy even before puberty. Echocardiography shows that Mybpc3 KO mice have left ventricular dilatation, increased ventricular wall thickness, and a significant decline in systolic function.

Figure 2: Left panel: Echocardiographic B-mode images; Right panel: M-mode echocardiographic parameters in different ages.
In addition, ECG monitoring reveals that Mybpc3 knockout induces arrhythmias and ventricular conduction block, characterized by a prolonged QT interval, increased Q-wave amplitude, and ST-segment elevation.
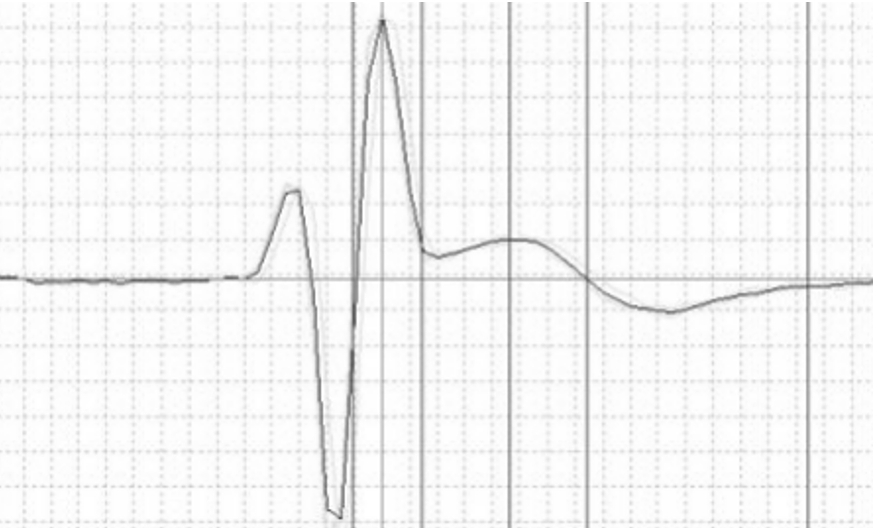
Figure 3: ECG of Mybpc3 mice showing ST-segment elevation phenotype.
Subsequent pathological analysis revealed that the myocardial tissue of 3-week-old Mybpc3 KO mice exhibited disorganized sarcomere structure, inflammatory cell infiltration, and significant collagen deposition.

Figure 4: Hematoxylin-Eosin (H&E) staining

Figure 5: Picrosirius Red (PSR) staining
Myh6 R404Q Concentric Hypertrophic Cardiomyopathy Model
The Myh7 R403Q mutation is frequently observed in human HCM, and this mutation reduces the binding affinity between Myh7 and Mybpc3[5]. To simulate the clinical pattern of Myh7 R403Q mutation, the arginine at amino acid position 404 of murine Myh6 was mutated to glutamine. This is because Myh6 in the mouse heart has similar functions to human Myh7 and exhibits high amino acid sequence homology.
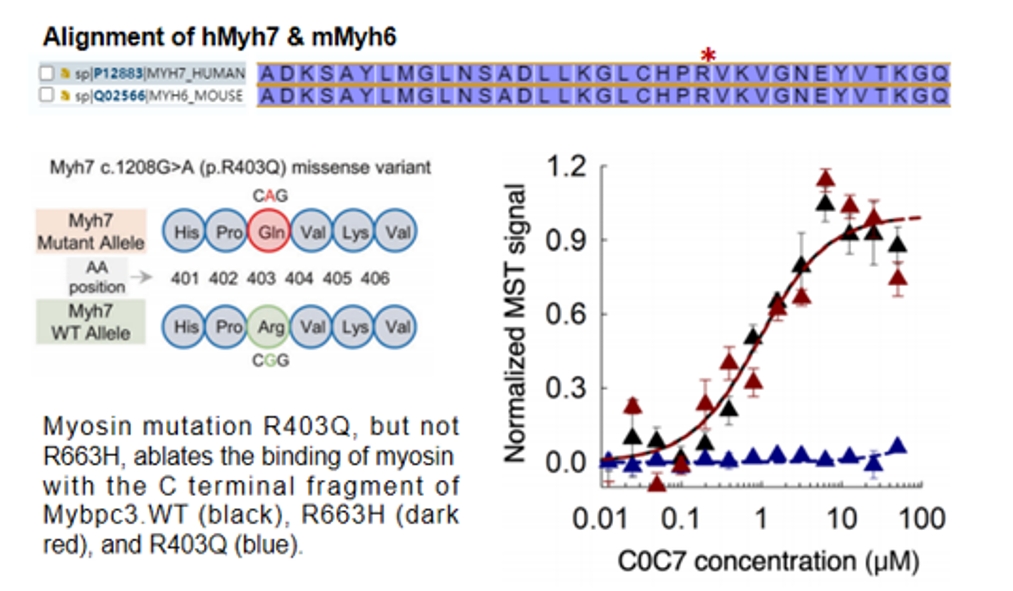
Reference: Sci Adv. 2020 Apr; 6(14): eaax0069.
Figure 6: Upper panel: Amino acid sequences around the mutation sites of human Myh7 and mouse Myh6; Lower panel: The R403 mutation in humans significantly reduces the binding affinity between the C-terminals of Myh7 and Mybpc3.
Myh6 R404Q heterozygous mice exhibit a concentric hypertrophic cardiomyopathy phenotype. Echocardiography shows that Myh6 R404Q mice exhibit reduced left ventricular volume, increased ventricular wall thickness, slightly improved systolic function, but decreased cardiac output.
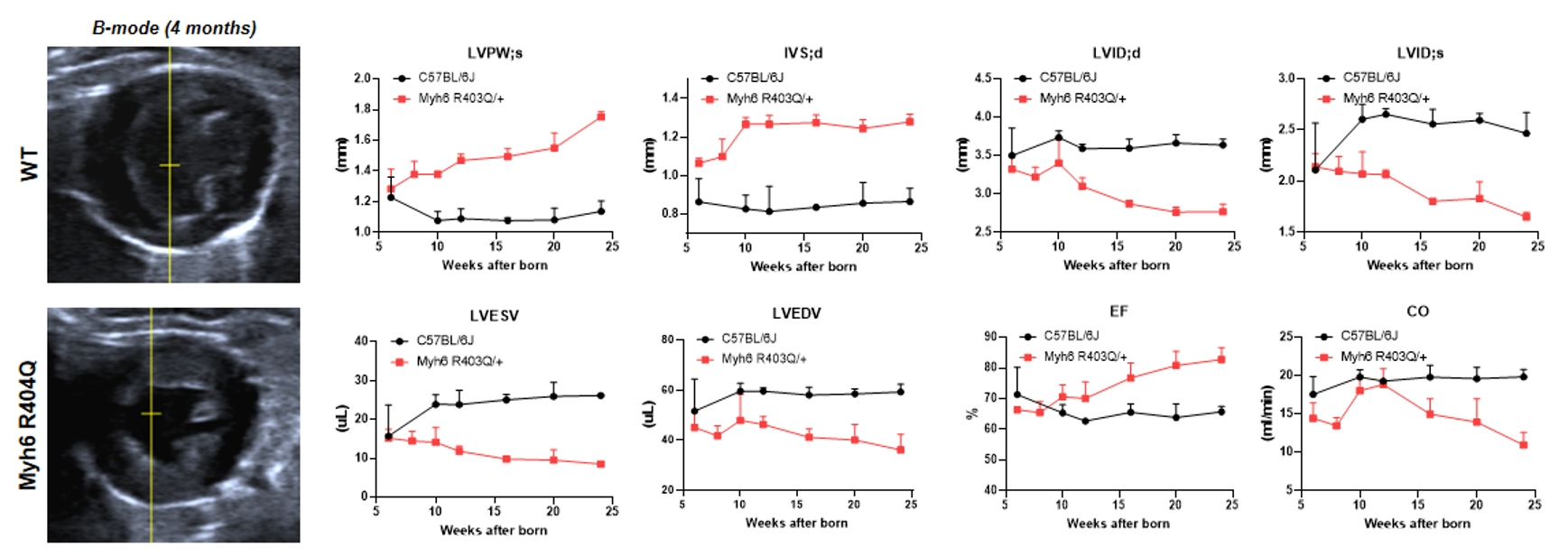
Figure 7: Left panel: Echocardiographic B-mode images; Right panel: M-mode echocardiographic parameters in different ages.
By faithfully simulating distinct HCM subtypes, GemPharmatech’s models allow researchers to:
Investigate underlying disease mechanisms
Test small molecules, biologics or gene therapies targeting sarcomeric proteins
Accelerate the translation of novel therapeutic strategies
Hypertrophic cardiomyopathy remains a leading cause of sudden cardiac death and chronic heart failure, with limited treatment options. By leveraging insights genetic insights from MYBPC3 and MYH6 mutations, GemPharmatech has developed two clinically relevant mouse models that mirror the progression of human disease. These models provide powerful research tools that help advance the discovery and validation of new HCM therapies.
References
1. Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015;65:1249-54. 10.1016/j.jacc.2015.01.019
2. Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995;92:785-9.
3. Luis R Lopes, Carolyn Y Ho et al. Genetics of hypertrophic cardiomyopathy: established and emerging implications for clinical practice. Eur Heart J. 2024;45(30):2727.
4. Julie Hathaway, Krista Heliö et al. Diagnostic yield of genetic testing in a heterogeneous cohort of 1376 HCM patients. BMC Cardiovasc Disord . 2021 Mar 5;21(1):126.
5. Saswata S Sarkar, Darshan V Trivedi et al. The hypertrophic cardiomyopathy mutations R403Q and R663H increase the number of myosin heads available to interact with actin. Sci Adv. 2020 Apr 3;6(14):eaax0069.


