Shaping the Future of In Vivo Genomic Editing: GemPharmatech’s Next-Generation NCG Portfolio
From Ex Vivo to In Vivo: A Paradigm Shift in Gene Therapy
For decades, gene therapy relied heavily on ex vivo modification. Patient primary cells were collected , engineered in the lab, and reinfused back into patients. This method produced major breakthroughs, but it came at a high cost: complex logistics, high treatment expenses, and risks associated with conditioning regimens.
Moving from ex vivo to in vivo genomic engineering is one way to circumvent these challenges. Therapies that directly edit the patient cells inside the body would eliminate the need forcell harvest and conditioning, dramatically simplifying the treatment and making gene therapy more widely accessible.
Breakthroughs in Delivery
Early efforts used viral vectors for in vivo editing, but safety concerns limited their impact. The emergence of lipid nanoparticles (LNPs), previously proven on a global scale with mRNA vaccines, opened a new frontier. These delivery vehicles offered transient, precise, and safer transport of gene editors such as CRISPR-Cas or base editors directly to target cells in the body.
Yet one major hurdle remained: validating these strategies in vivo in a way that faithfully recapitulates the human hematopoietic environment.
GemPharmatech’s Next-Generation NCG Portfolio: Enabling True In Vivo Validation
This is where GemPharmatech’s next-generation NCG portfolio has become essential. Built on the foundation of GemPharmatech’s NCG, a T, B and NK cell severely immunodeficient mouse, these next generation models, created in-house by GemPharmatech’s team of experts, support robust engraftment of human tissue including PBMCs and HSCs.
Among them, the NCG-X strain has played a pivotal role in recent breakthroughs such as the 2025 study published in Nature Biomedical Engineering. Researchers demonstrated that LNP-delivered base editor mRNA could precisely edit the HBG gene promoter in humanized mice, activating fetal hemoglobin and offering a conditioning-free, transplantation-free therapeutic path for β-thalassemia and sickle cell disease.
The success of this work underscores the critical strengths of GemPharmatech’s NCG portfolio:
Faithful human hematopoietic niche: supporting long-term survival and differentiation of human cells.
In vivo gene editing validation: enabling direct testing of mRNA/LNP systems with human HSCs.
Long-term efficacy and safety assessment: permitting comprehensive monitoring of multilineage reconstitution, chimerism, and off-target safety profiles.
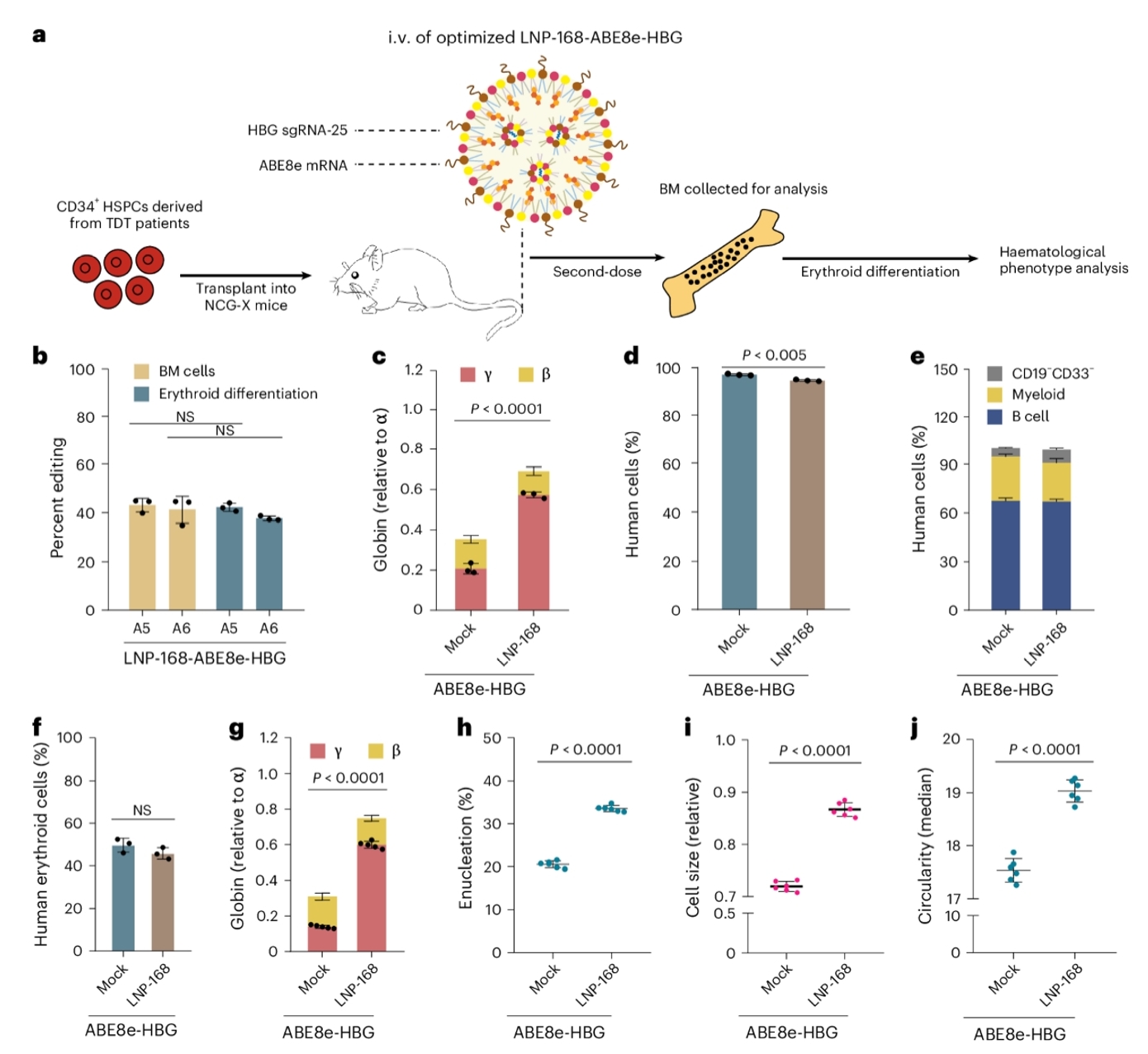
Fig.1 LNP-ABE8e-HBG achieves therapeutic base editing in hematopoietic stem and progenitor cells within NCG-X mice engrafted with hCD34+ cells from a β-thalassemia patient.
Why this Matters: A New Chapter in Gene Therapy
As a result of the rise of in vivo editing tools and the availability of next-generation NCG humanized models, gene therapy is entering a new era where translation from lab to clinic can happen faster and with greater confidence.
By bridging the gap between historical ex vivo approaches and emerging in vivo strategies, GemPharmatech’s NCG portfolio is accelerating the field toward therapies that are not only more effective and accessible but potentially safer. What once required complex stem cell harvests and conditioning regimens can now be envisioned as a “one-step” therapy delivered intravenously.
Partnering for the Future of In Vivo Genomic Engineering
GemPharmatech is committed to partnering with biotech and pharmaceutical innovators to accelerate the development of in vivo gene editing therapies. With the world’s largest portfolio of genetically engineered mouse strains, including next-generation NCG models purpose-built for hematopoietic research, we provide a trusted preclinical bridge from discovery to clinical translation.
Whether your program focuses on monogenic blood disorders, immune modulation, or next-gen delivery systems, GemPharmatech’s integrated preclinical services and advanced humanized models ensure that your research is validated and positioned for real-world success.
Reference: https://www.nature.com/articles/s41551-025-01480-y
The NCG-X is licensed to Charles River Laboratories for breeding and distribution


