The growing intersection between oncology and immunology is opening new therapeutic avenues, particularly in the treatment of autoimmune diseases. Recently, cell or antibody-based therapies (such as CAR-T and bispecific antibodies) originally developed for B-cell malignancies have garnered attention for their potential to treat autoimmune diseases. This shift highlights a broader trend in drug repurposing efforts, where cancer immunotherapies are being evaluated for new applications in the autoimmune disease space.
At the core of this approach is the pathological role B-cells play in driving autoimmune diseases. Aberrant B-cell activation against self-antigens and subsequent autoantibody production are key to the pathogenesis of many autoimmune diseases. This mechanism plays a pivotal role in diseases such as systemic lupus erythematosus (SLE), multiple sclerosis (MS), myasthenia gravis (MG), and Sjögren's syndrome (SS). By targeting B-cells, therapies designed to eliminate cancerous clones in B-cell malignancies may also interrupt the autoimmune disease cascade, thus offering a new strategy for disease control and remission.
To support preclinical evaluation of these emerging therapies, GemPharmatech has established a diverse portfolio of autoimmune disease models suitable for evaluating B-cell targeted interventions. This portfolio includes genetically engineered mouse models, induced disease mouse models, and human immune system-reconstituted mouse models. Collectively, they enable the investigation of B-cell mediated pathology across multiple autoimmune diseases such as SLE, MS, MG, and Sjögren’s syndrome.
By combining model diversity with in vivo study support, GemPharmatech enables researchers to generate high-confidence data on B-cell depletion strategies across multiple autoimmune indications, accelerating preclinical validation and informing translational decision-making.
1. Systemic Lupus Erythematosus (SLE)
Systemic lupus erythematosus is a complex autoimmune disorder characterized by immune system dysregulation leading to the production of pathogenic autoantibodies. In affected individuals, dysregulated B-cell activation and tolerance breakdown results in the generation of autoantibodies that target nuclear and cytoplasmic self-antigens. These autoantibodies form immune complexes that deposit in various tissues, activating the complement cascade and inflammatory damage. Clinically, SLE is characterized by multisystem involvement, affecting the skin, joints, kidneys, hematopoietic system, and other tissues.
1.1 Spontaneous SLE Models: B6-hBAFF and B6-hCD20/hBAFF
BAFF (B-cell activating factor, also known as BLyS) is a key cytokine that supports the maturation and survival of B-cells, including autoreactive B-cells. Excessive BAFF expression enhances the pool of autoreactive B cells, ultimately driving autoimmune responses. Systemic lupus erythematosus (SLE) patients typically exhibit BAFF overexpression, which has been validated as a critical therapeutic target in such autoimmune diseases.
To facilitate preclinical evaluation of B-cell-targeted therapies for SLE, GemPharmatech has successfully developed genetically humanized mice that express hBAFF. Compared to wild-type mice, hBAFF-transgenic mice show significantly elevated serum levels of anti-dsDNA IgG, IgA, and IgM antibodies, increased B cell proportions in the spleen and lymph nodes, and proteinuria (manifested as elevated urinary Microalbumin/creatinine ratios) in older mice, mimicking the pathogenic mechanisms and clinical manifestations of SLE in humans. These models have successfully enabled efficacy evaluation of B-cell-targeted drugs such as belimumab (Figure 1).
To further support preclinical development of multi-target B-cell-related drugs, we have developed a series of dual-target humanized mice based on the BAFF-humanized spontaneous SLE model, such as hAPRIL/hBAFF and hCD20/hBAFF dual-target humanized mice. The phenotype of those models are consistent with the spontaneous SLE phenotype observed in B6-hBAFF (Figure 2).
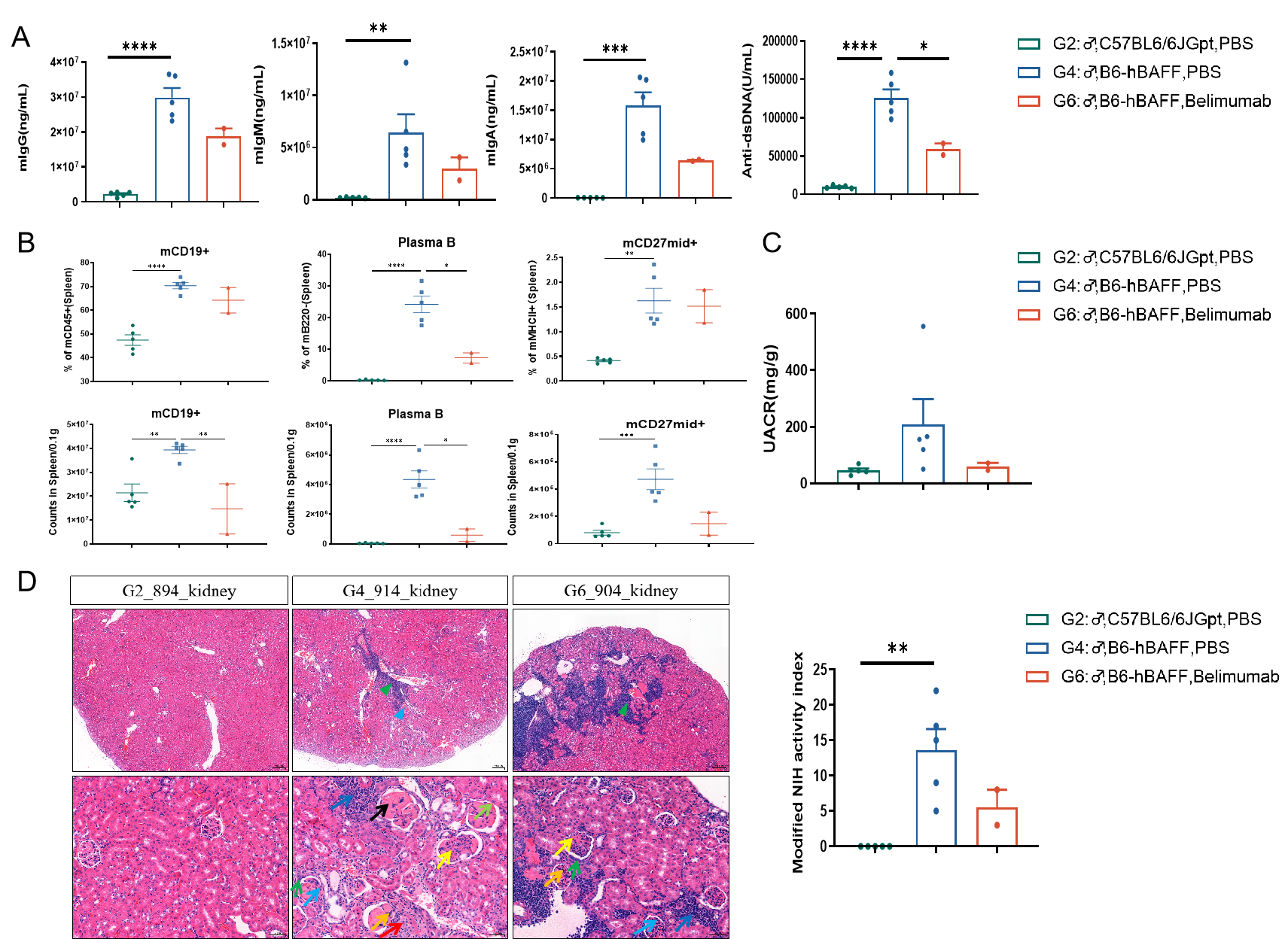
Figure 1: Efficacy Evaluation of Belimumab in B6-hBAFF Humanized Mouse SLE Model
Following 16 weeks of belimumab treatment in BAFF humanized mice, serum levels of anti-dsDNA antibodies, IgG, IgA, and IgM were reduced (A). The number and proportion of CD19+B cells, plasma cells, and CD27+ memory B cells in splenocytes decreased (B); and the urinary Microalbumin/creatinine ratio (UACR) and renal HE pathology scores (C-D) were reduced after 20 weeks of treatment. Data shown as Mean ± SEM; *: P<0.05;**: P<0.01; ***: P<0.001; ****:P<0.0001: vs Group 4.
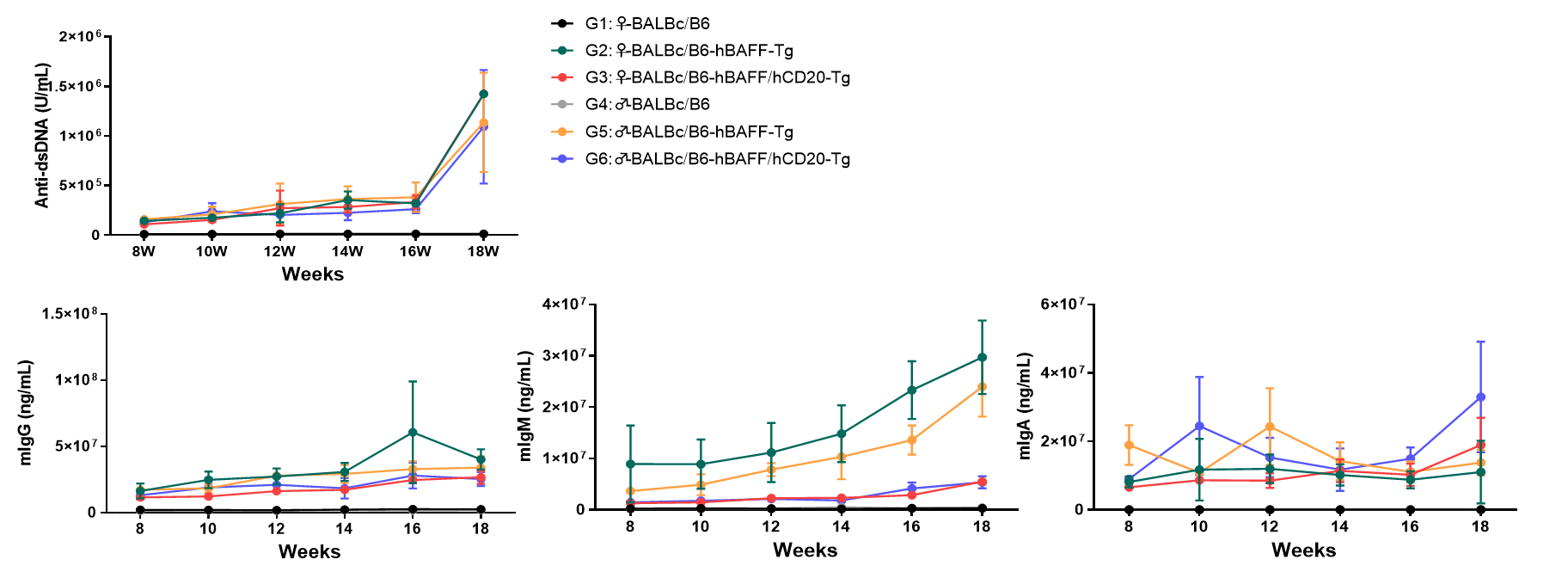
Figure 2: SLE Phenotype Analysis of B6-hCD20/hBAFF Humanized Mice
Consistent with the spontaneous SLE phenotype of B6-hBAFF mice, 6~18-week-old hCD20/hBAFF dual-target humanized mice exhibited significantly elevated serum levels of anti-dsDNA antibodies, IgG, IgA, and IgM.
1.2 Immune-Reconstituted SLE Models
Considering the mechanism of action of biologics such as CD3/CD19 bispecific or multispecific antibodies, careful selection of animal models for the preclinical evaluation of these treatment modalities is warranted. For such purposes, humanized animal models—particularly immune system-humanized SLE models are recommended. GemPharmatech has established two robust preclinical evaluation systems for immune system-humanized autoimmune models: the first is a PBMC-NCG mouse model reconstituted with SLE patient-derived peripheral blood mononuclear cells (PBMCs) and the second is based on a huHSC-NCG-M human immune system reconstituted mice induced with toll-like receptor agonist (Figure 3). These immune-reconstituted SLE models effectively recapitulate clinical phenotypes of human SLE patients, characterized by significantly elevated serum levels of anti-dsDNA antibodies and IgG, as well as glomerular deposition of human IgG (Figure 4).
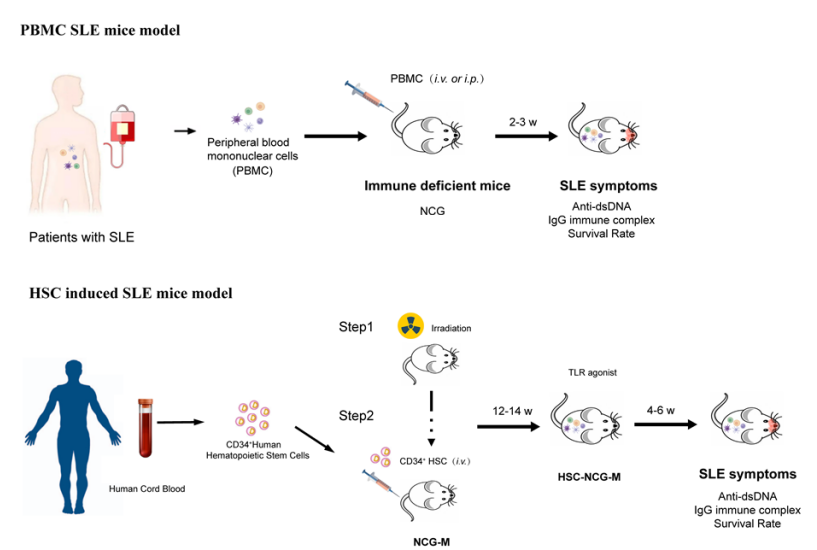
Figure 3: Non-Clinical Evaluation Process for Drugs Based on SLE Patient-Derived-PBMC-NCG and HSC-NCG-M Immune-Reconstituted SLE Model
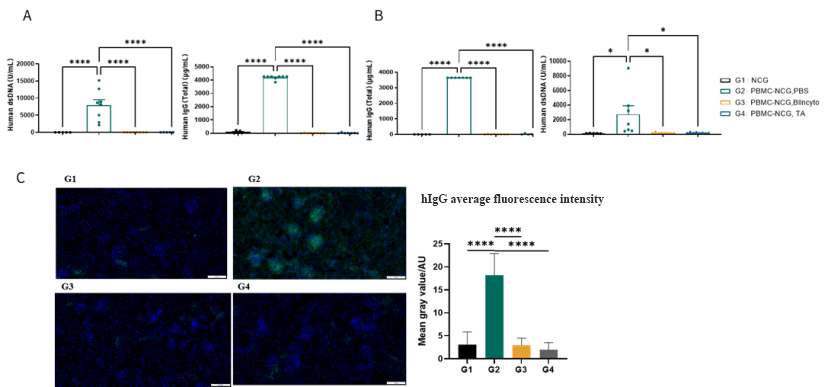
Figure 4: Non-clinical Pharmacodynamic Evaluation of Blincyto in SLE Patient-Derived-PBMC-NCG Mouse Model
We developed a novel SLE mouse model through the intraperitoneal injection of SLE patient-derived peripheral blood mononuclear cells (PBMCs) into immunodeficient NCG mice. Human anti-dsDNA antibodies and total IgG were detected in plasma at two weeks (A) and four weeks (B) post-injection. IgG deposition was observed in the kidneys (C). Treatment with Blincyto and another TCE test article (TA) reduced the levels of antibody and renal IgG deposition. Data shown as Mean ± SEM; *: P<0.05; ****: P<0.0001: vs Group 2.
2. Multiple Sclerosis
Multiple sclerosis (MS) is a chronic autoimmune disease in which the immune system mistakenly attacks myelin, the protective insulating sheath that surrounds nerves in the brain and spinal cord, resulting in progressive neurological dysfunction. There is no cure for MS and current treatments are focused on reducing the frequency and severity of attacks as well as delaying disease progression. To help test new treatment methods, the classical Experimental Autoimmune Encephalomyelitis (EAE) serves as a critical animal model for multiple sclerosis (MS) research. There are several ways to induce EAE in mice, and a common method includes the use of Myelin Oligodendrocyte Glycoprotein (MOG). In MOG35-55 induced model, the disease is primarily mediated by specifically sensitized CD4+ T cells, while B cells remain inactive. In contrast, in the EAE model induced with MOG1-125, B cells are activated, functioning as antigen-presenting cells to promote cytokine production and the differentiation of antibody-producing plasma cells (Figure 5).
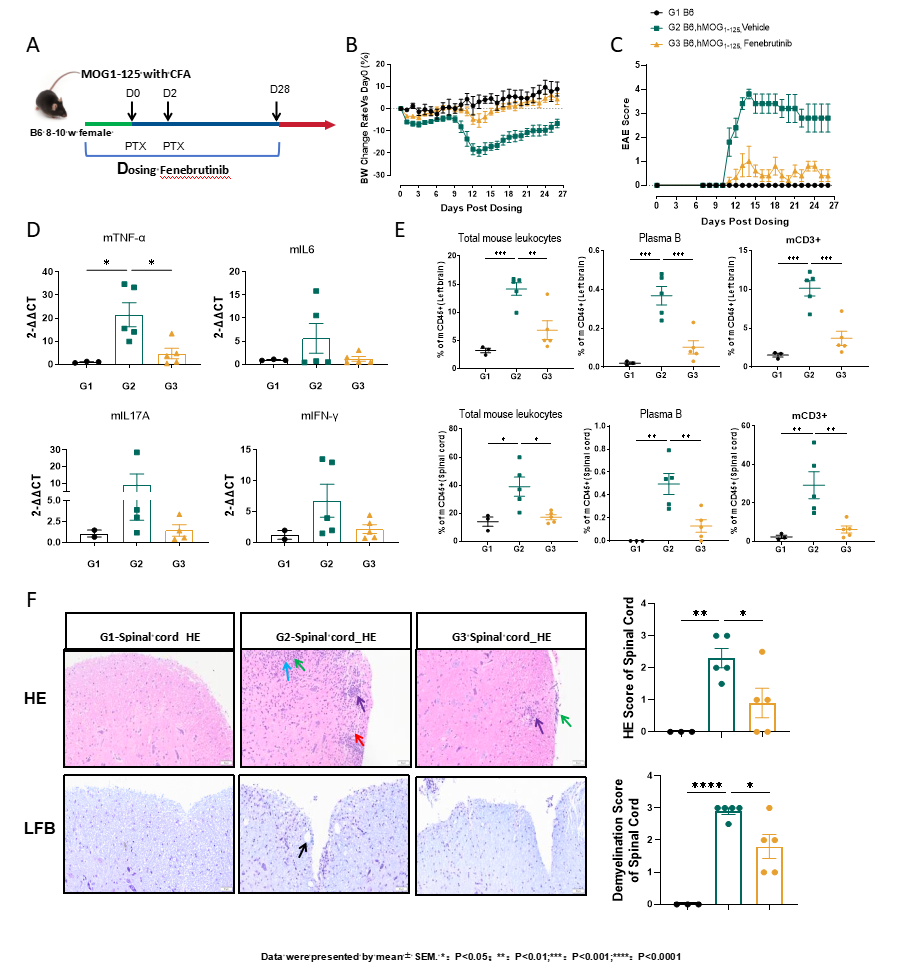
Figure 5: Efficacy valuation of Fenebrutinib in MOG1-125 induced EAE model
Efficacy evaluation of Fenebrutinib in MOG1-125 induced EAE model strategy was performed as shown (A). Changes in body weight (B) and EAE scores (C),mRNA levels (D), immune cell in the brain (Top) and spinal cord (bottom) (E),HE score and the demyelination score of spinal cord (F) were all alleviated following Fenebrutinib treatment. Data shown as Mean ± SEM; *: P<0.05;**: P<0.01; ***: P<0.001: vs Group 2.
3. Myasthenia Gravis
Myasthenia gravis (MG) is an autoimmune disorder where autoantibodies disrupt neuromuscular junction (NMJ) signaling, impairing the communication between nerves and muscles. This disease is primarily mediated by pathogenic antibodies directed against the postsynaptic membrane, including acetylcholine receptor (AChR) antibodies, muscle-specific kinase (MuSK) antibodies, and low-density lipoprotein receptor-related protein 4 (LRP4) antibodies. These antibodies induce the loss of AChR at the NMJ, impairing neuromuscular transmission and leading to clinical manifestations of fatigue, muscle weakness, and in severe cases, life-threatening complications. GemPharmatech has established two experimentally induced MG models that recapitulate clinical phenotypes of the disease (Figures 6 and 7) which can be used to assess the efficacy of any new therapeutic modality.
3.1 AChRα 97-116-Induced Experimental Autoimmune Myasthenia Gravis (EAMG) Model
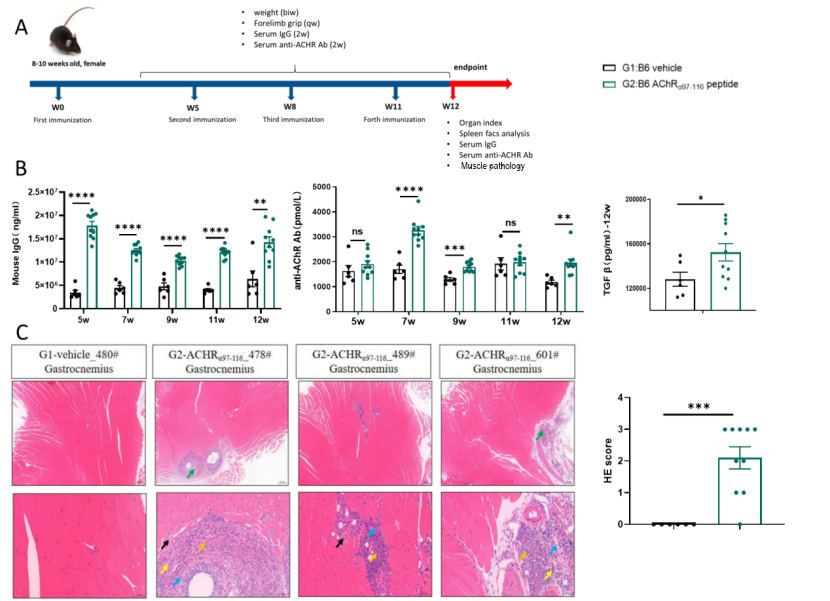
Figure 6: AChRα 97-116-Induced Experimental Autoimmune Myasthenia Gravis (EAMG) Model
(A). After induction with AChRα 97-116, mice in the model group exhibited elevated serum levels of total IgG, anti-AChR antibodies, and TGF-β (B). Pathological changes in the gastrocnemius muscle included muscle fiber degeneration and necrosis, inflammatory cell infiltration, and fibrous tissue hyperplasia (C). Data shown as Mean ± SEM; *: P<0.05;**: P<0.01; ***: P<0.001: vs Group 1.
3.2 MG Patient Derived PBL-NCG Model
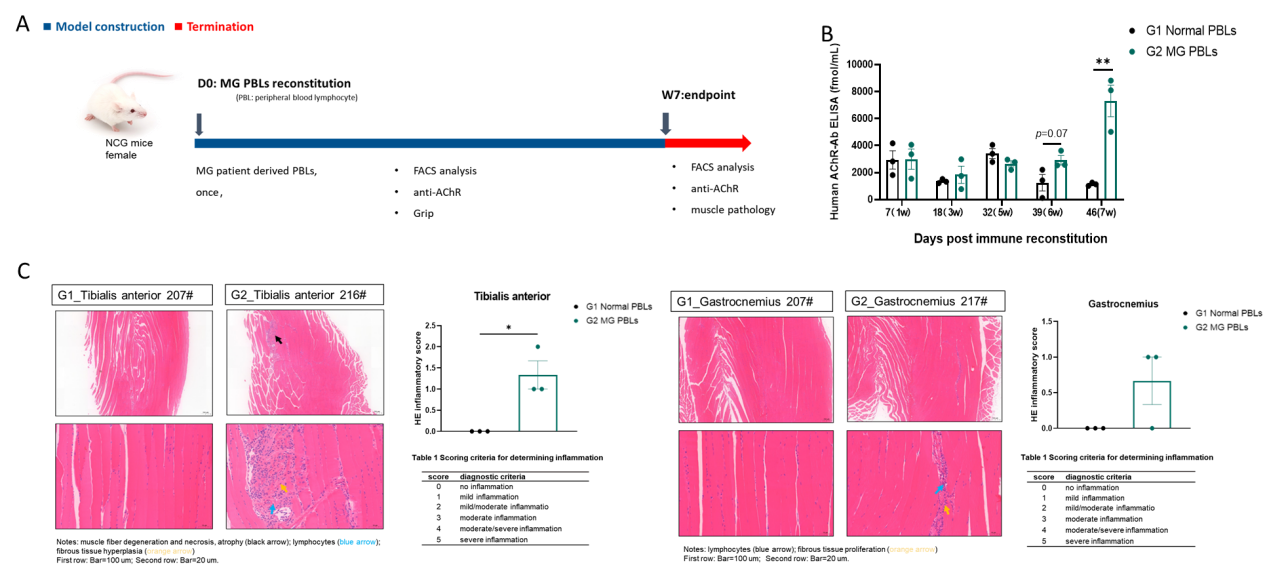
Figure 7: MG patient derived PBL-NCG model
We developed a novel mouse model for MG by intraperitoneal injection of MG patient derived PBL into immunodeficient NCG mice (A), resulting in elevated levels of anti-AChR antibody levels (B). Inflammatory cell infiltration in muscle was also increased as evidenced by HE staining (C). Data shown as Mean ± SEM; *: P<0.05;**: P<0.01;****: P<0.0001: vs Group 1.
4. Sjögren’s Syndrome
Sjögren's syndrome (SS) is an autoimmune disorder that primarily affects the exocrine glands, notably the salivary and lacrimal glands. This leads to hallmark clinical features such as dry mouth (xerostomia) and dry eyes (xerophthalmia). Sjögren's syndrome can also involve other organs and systems, causing various symptoms such as joint pain, fatigue, and difficulties in swallowing. The Sjogren's syndrome model induced by salivary gland proteins is a classic animal model used for evaluating the preclinical efficacy of drugs for treating Sjogren's syndrome (Figure 8).
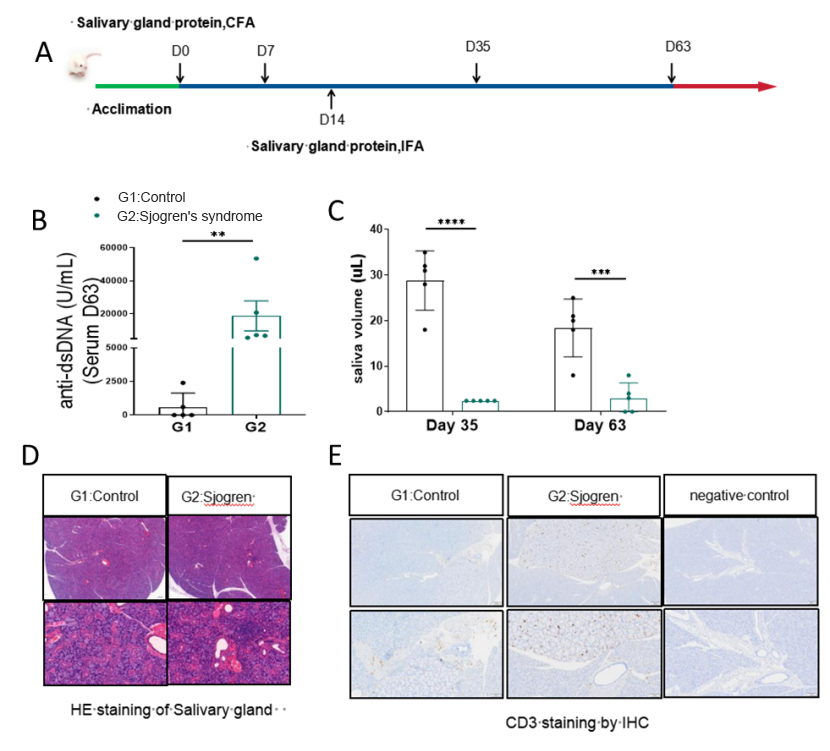
Figure.8 Salivary gland protein-induced mouse SS model
The salivary gland protein induced model strategy was performed as shown (A). After immunizing Balb/c mice for 35 or 63 days, the level of anti-dsDNA in the serum increased in model group (B) while salivary flow rate of the mice in the model group decreased (C). The results of HE pathological staining of the salivary glands indicated that inflammatory cells, including CD3+ T cells infiltrated the salivary gland tissues in the model group (D and E). Data shown as Mean ± SEM; **: P<0.01; ***: P<0.001; ****: P<0.0001: vs Group 1.


