Unlocking the Potential of Antibody-Drug Conjugates: GemPharmatech’s Comprehensive In Vitro Evaluation Platform
Antibody-drug conjugates (ADCs) represent a rapidly advancing therapeutic class for both solid tumors and hematological malignancies. Unlike conventional monoclonal antibodies, ADCs combine a tumor-targeting antibody with a highly potent cytotoxic payload, enabling selective drug delivery to cancer cells.
At GemPharmatech, we provide integrated end-to-end preclinical solutions to support ADC development. These solutions center around an advanced in vitro platform as well as in vivo capabilities to support every step of ADC functional assessment, including:
Antigen expression and target validation
Binding affinity and specificity
Internalization efficiency
Cytotoxicity and dose response
Cell cycle and apoptosis induction
Bystander killing effects
Combination therapy assessment
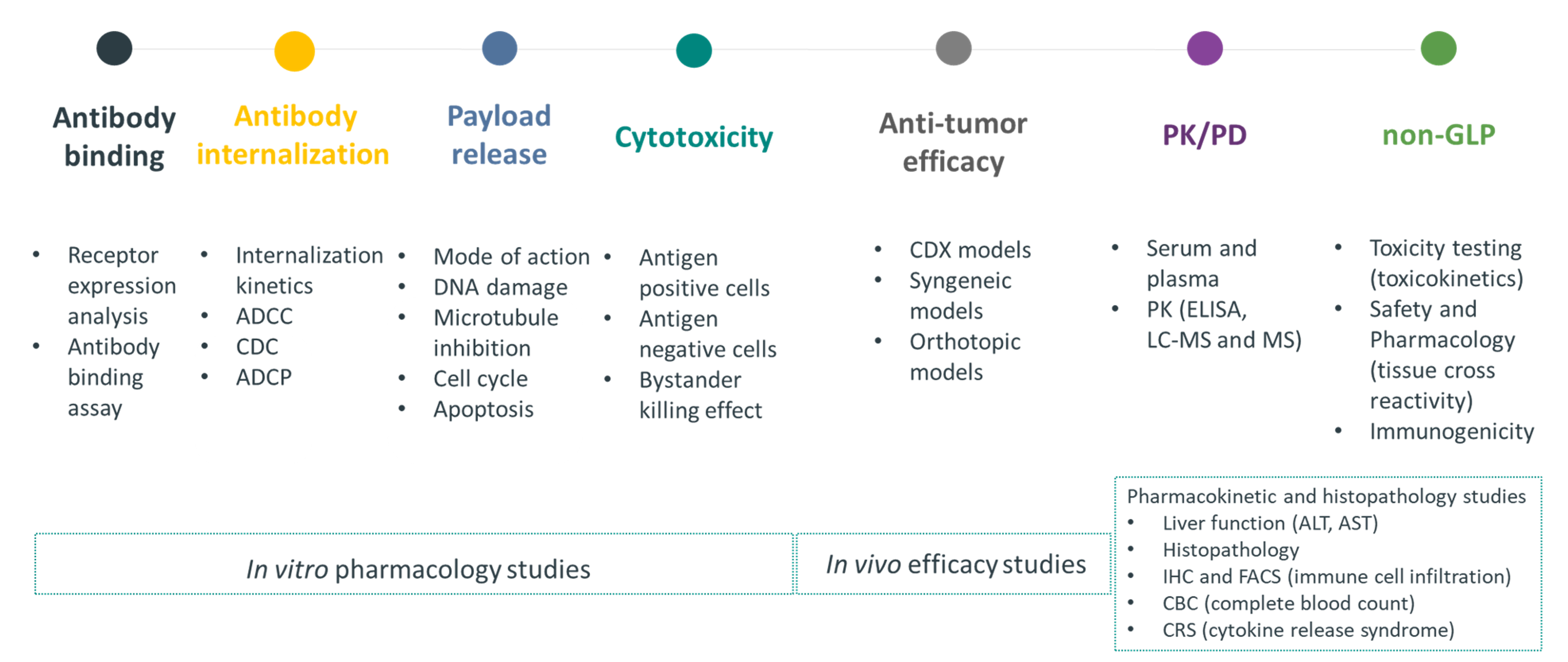
Figure 1. GemPharmatech in vitro service for ADC evaluation
Case Study 1: Antigen Expression Analysis
Objective: Assess c-Met and HER2 surface expression across 8 cell lines to determine suitability for ADC targeting
Method: Flow cytometric analysis
Result: Both c-Met and HER2 were widely distributed across tumor cells, highlighting opportunities for dual-targeted ADCs or combination therapies to enhance cancer treatment efficacy.
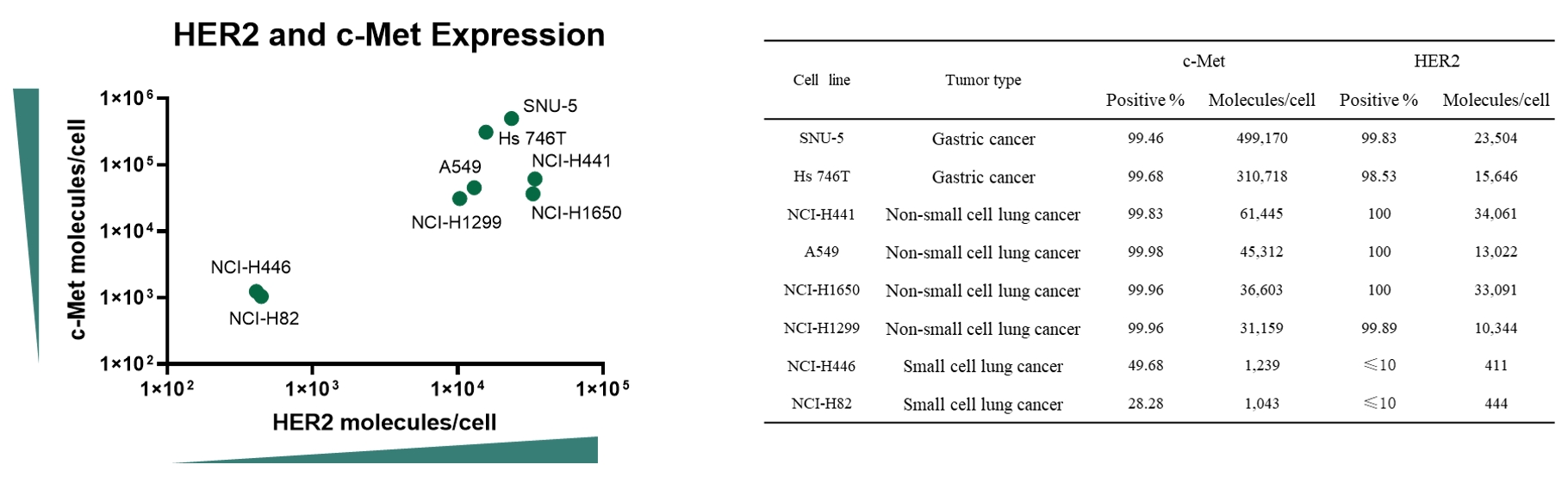
Figure 2. HER2 and c-Met surface expression across 8 cell lines
Case Study 2: Binding Affinity Assay
Objective: Compare binding of ABBV-399(c-Met ADC Drug) to c-Met with its parental antibody, Telisotuzumab
Method: Flow cytometric analysis
Result: ABBV-399 demonstrated robust binding across all three tumor cell lines, with binding characteristics similar to Telisotuzumab (c-Met antibody).
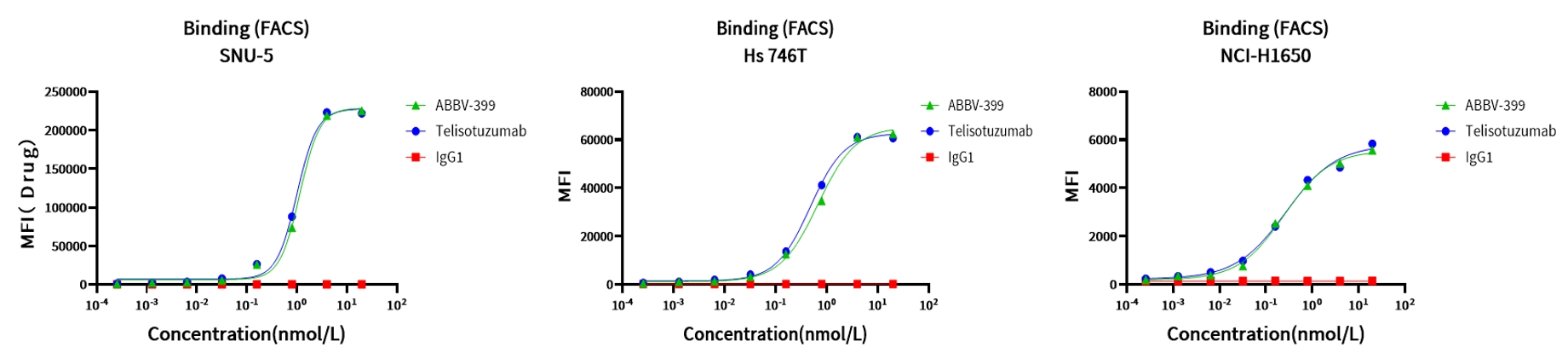
Figure 3. Binding assay of ABBV-399 in different cancer cell lines
Case Study 3: Cytotoxicity Screening
Objective: Identify tumor cell lines most susceptible to ABBV-399-mediated cytotoxicity
Method: CellTiter-Glo (CTG) assay
Result: ABBV-399 induced potent cytotoxic activity against four tumor cell lines, with its activity strongly correlated to the levels of c-Met overexpression. Notably, in SNU-5 cells, ABBV-399 exhibited significantly stronger anti-proliferative effects compared to its free cytotoxic payload (MMAE).
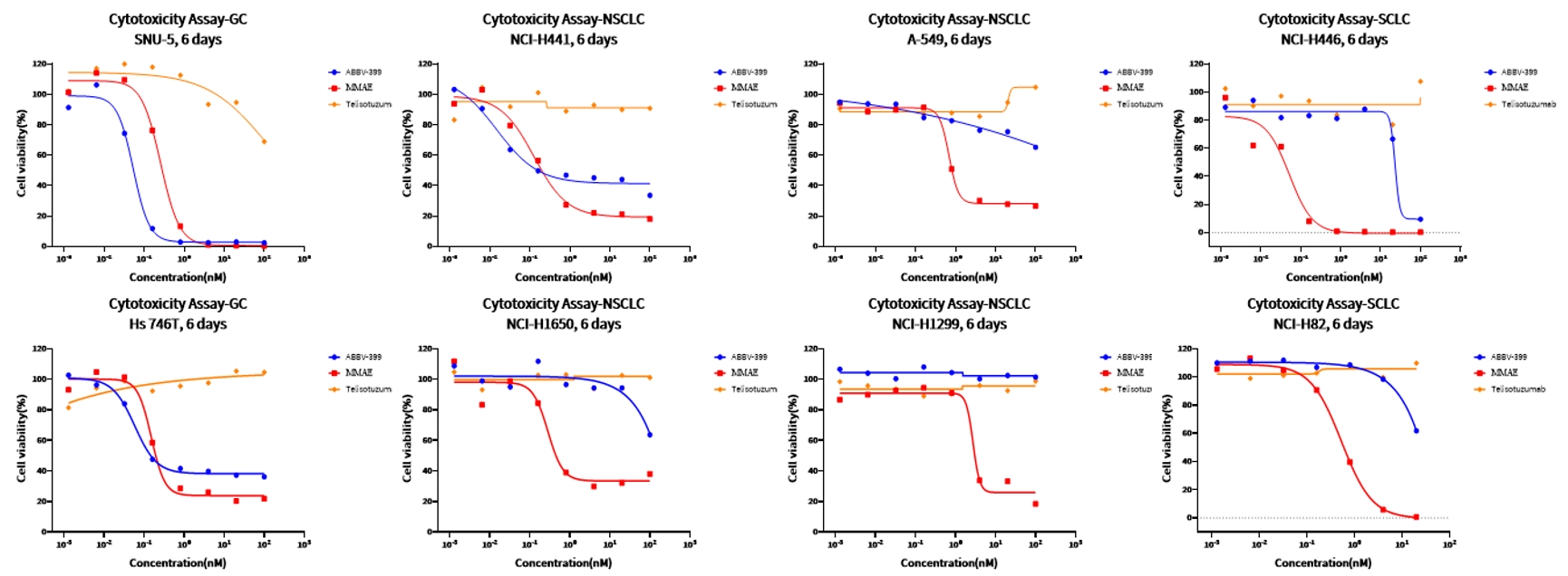
Figure 4. Dose-response curves of ABBV-399 against tumor cell lines
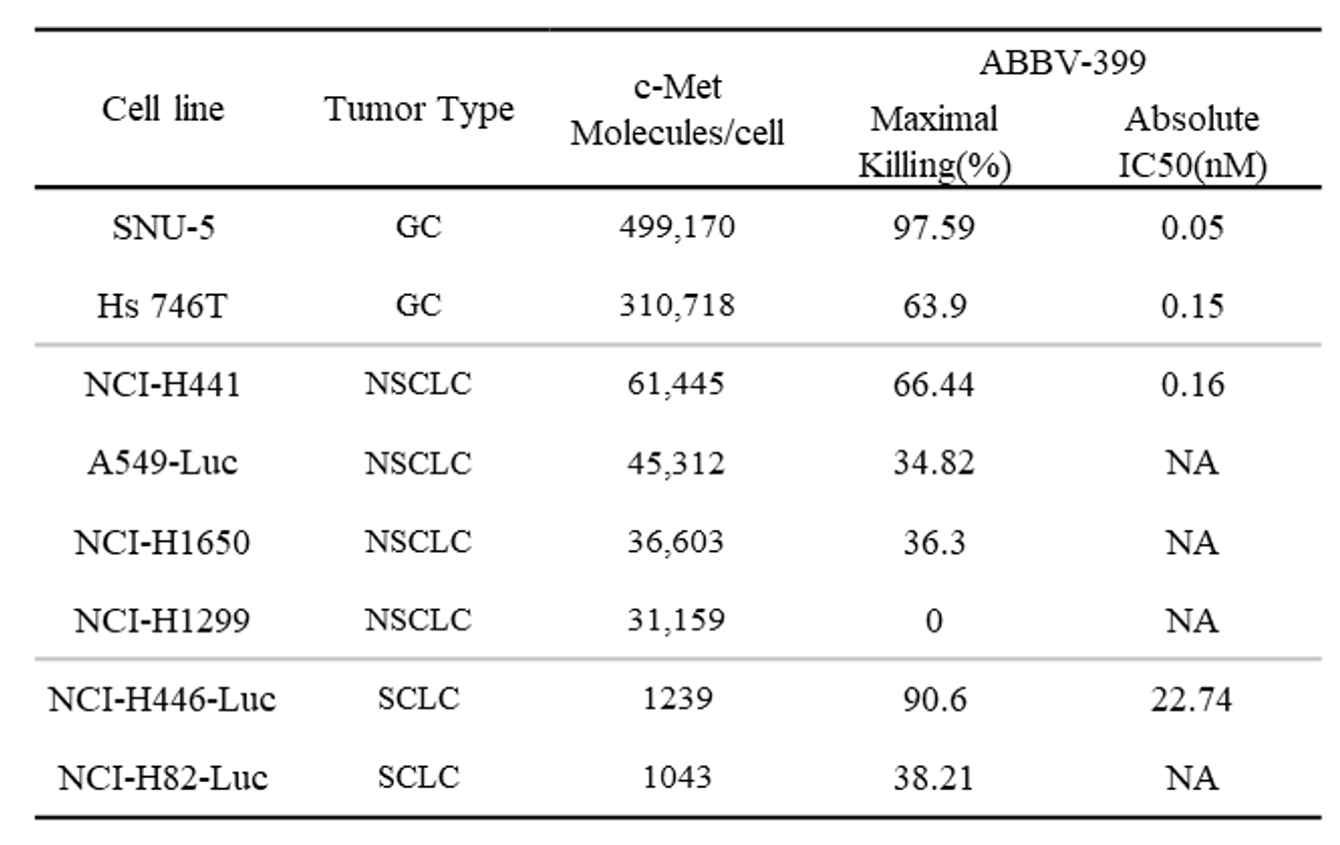
Table 1. c-Met expression on tumor cells in vitro and sensitivity to ABBV-399
Case Study 4: Internalization Assay
Objective: Evaluate the internalization of ABBV-399
Method: Flow cytometric analysis with pHrodo™ dye
Result: ABBV-399 demonstrated a time-dependent internalization effect similar to Telisotuzumab. Notably, low internalization signal in c-Met-positive NCI-H1650 cells, accounted for the absence of cytotoxicity despite binding.
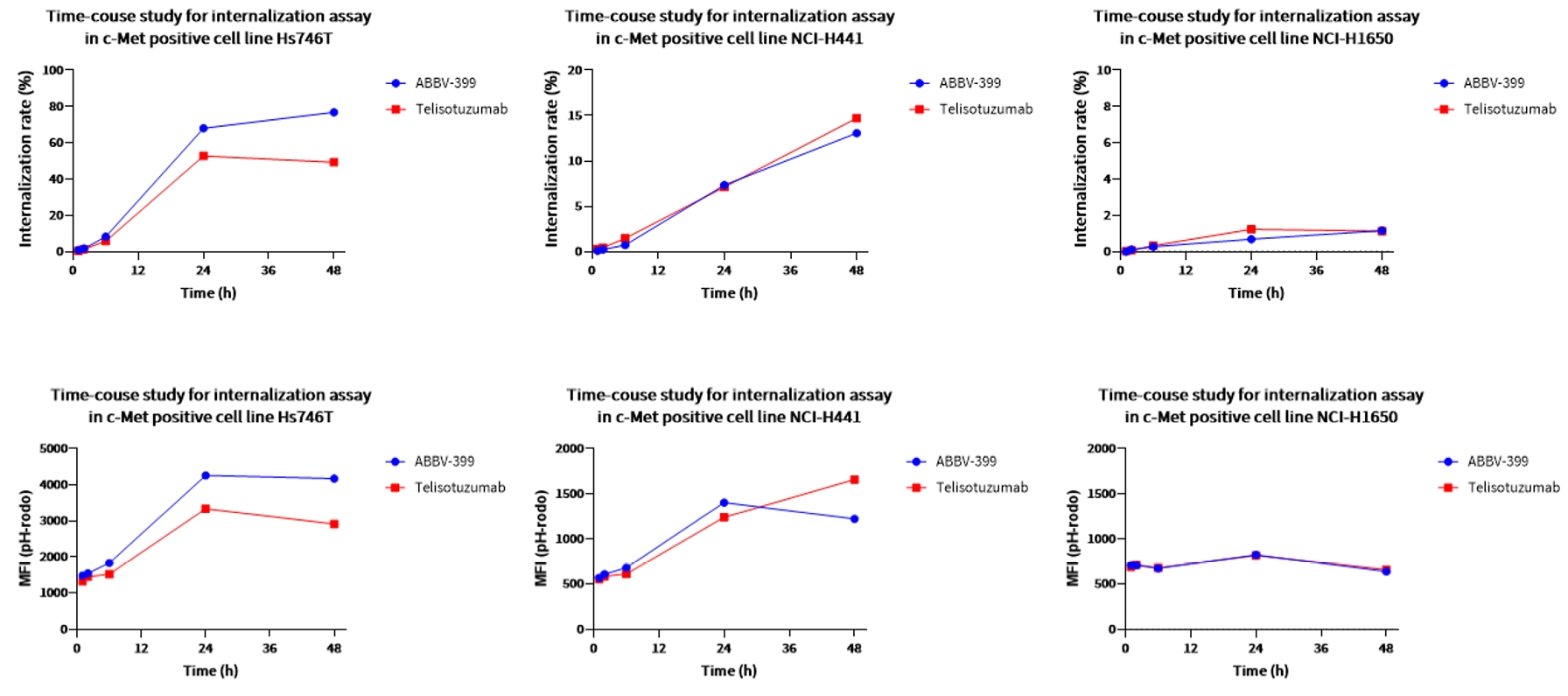
Figure 5. The internalization kinetics of ABBV-399
Case Study 5: Cell Cycle and Apoptosis Assay
Objective: Evaluate the effects of DS-8201a on SK-BR-3 cells
Method: Flow cytometric analysis
Result: Following treatment for 48 h, the proportion of S phase cells in the DS-8201a group was significantly increased compared with the control group, suggesting S-phase arrest via topoisomerase I inhibition (Figure 6). The proportion of apoptotic cells in DS-8201a group was also significantly increased compared with the control group, suggesting that DS-8201a induced cell apoptosis in a cell cycle-dependent or-independent manner (Figure 7).
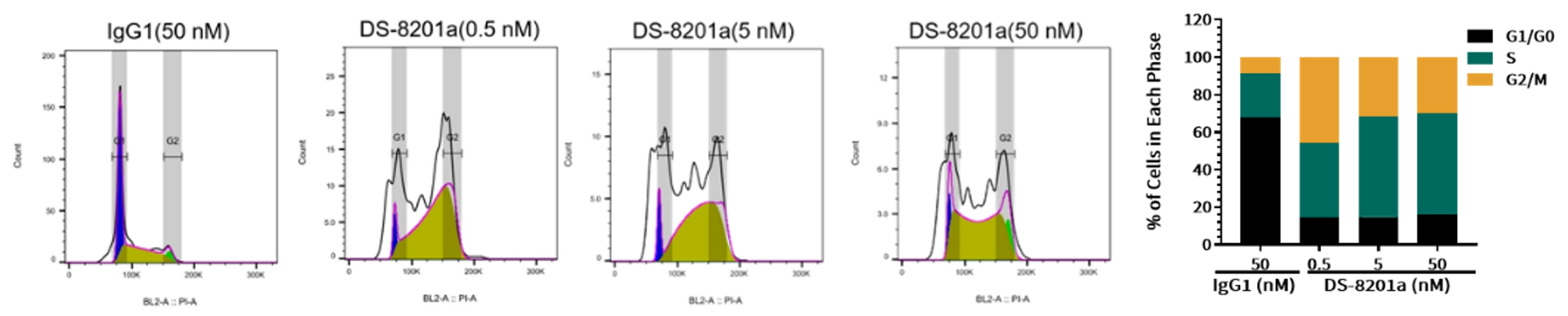
Figure 6. The effects of different concentrations of DS-8201a on the cell cycle of the target cell SK-BR-3
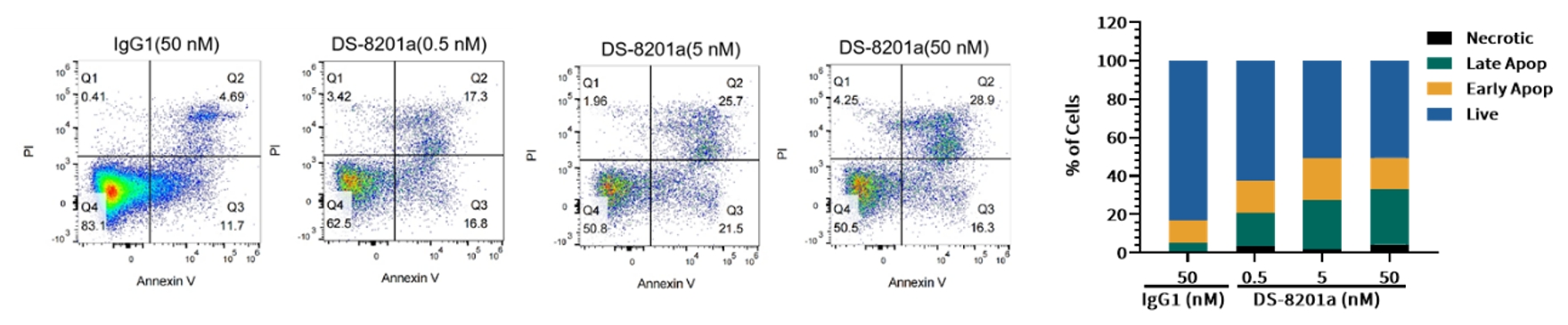
Figure 7. Apoptosis induced by DS-8201a in target cells SK-BR-3 at different concentrations
Case Study 6: Bystander Killing in Co-culture Conditions in vitro (FACS)
Objective: Assess the bystander effect of DS-8201a on HER2-positive and HER2-low tumor cells
Method: Flow cytometric analysis
Result: DS-8201a exerted direct cytotoxicity against HER2-positive SK-BR-3 cells and demonstrated bystander killing effects on antigen-negative MDA-MB-468 cells in coculture.
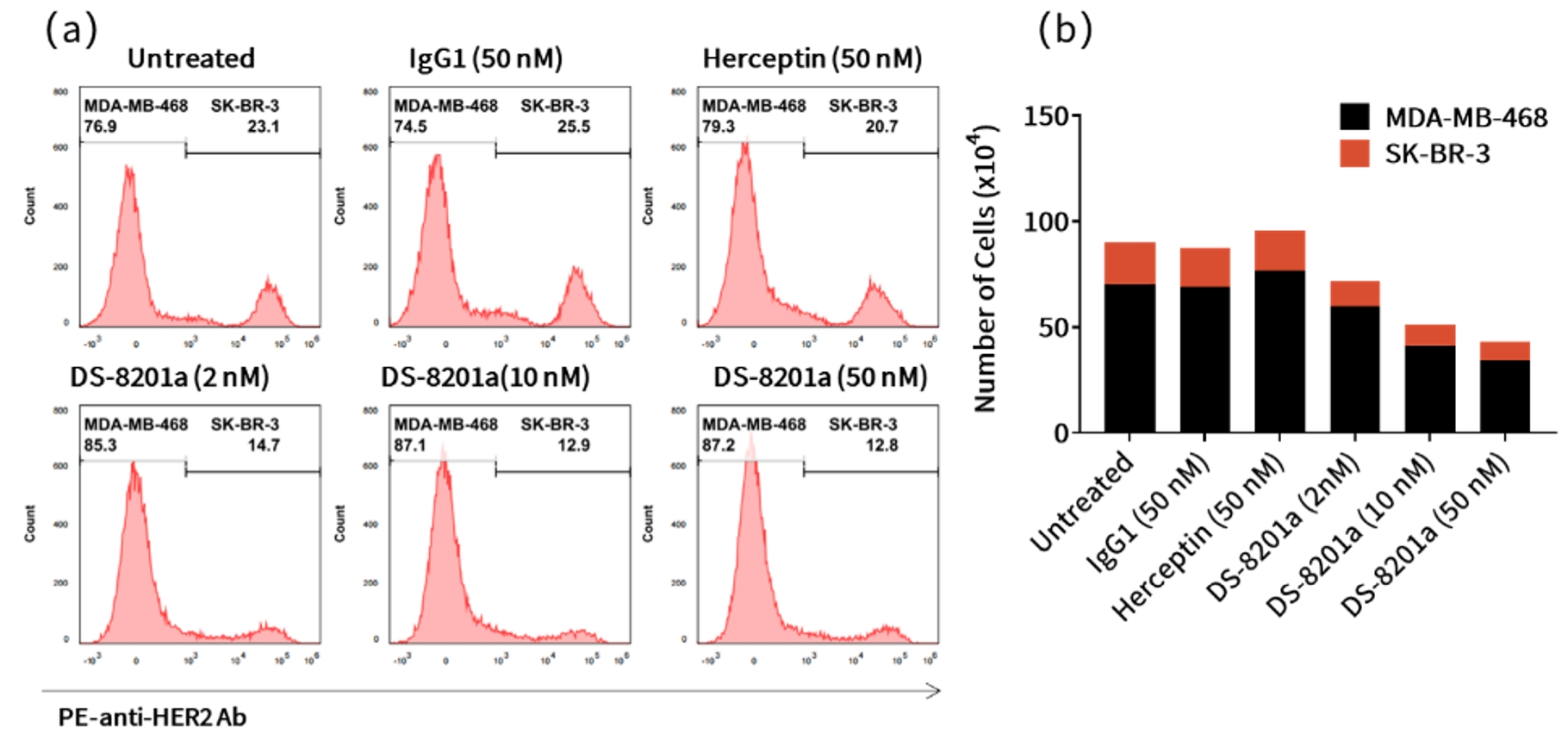
Figure 8. In vitro bystander killing ffect of ABBV-399. (a) Population analysis by flow cytometry. (b) Number of viable SK-BR-3 and MDA-MB-468 cells following DS-8201a treatment.
Case Study 7: Bystander Killing in Co-culture Conditions in vitro (Luciferase Reporter Cell)
Objective: Evaluate ABBV-399 bystander effects in mixed cultures of c-Met+ SNU-5 cells c-Met-low cells
Method: Co-culture with luciferase-labeled reporter cell
Result: ABBV-399 reduced the viability of c-Met-low reporter cells in a concentration-dependent manner, demonstrating a robust bystander killing effect.
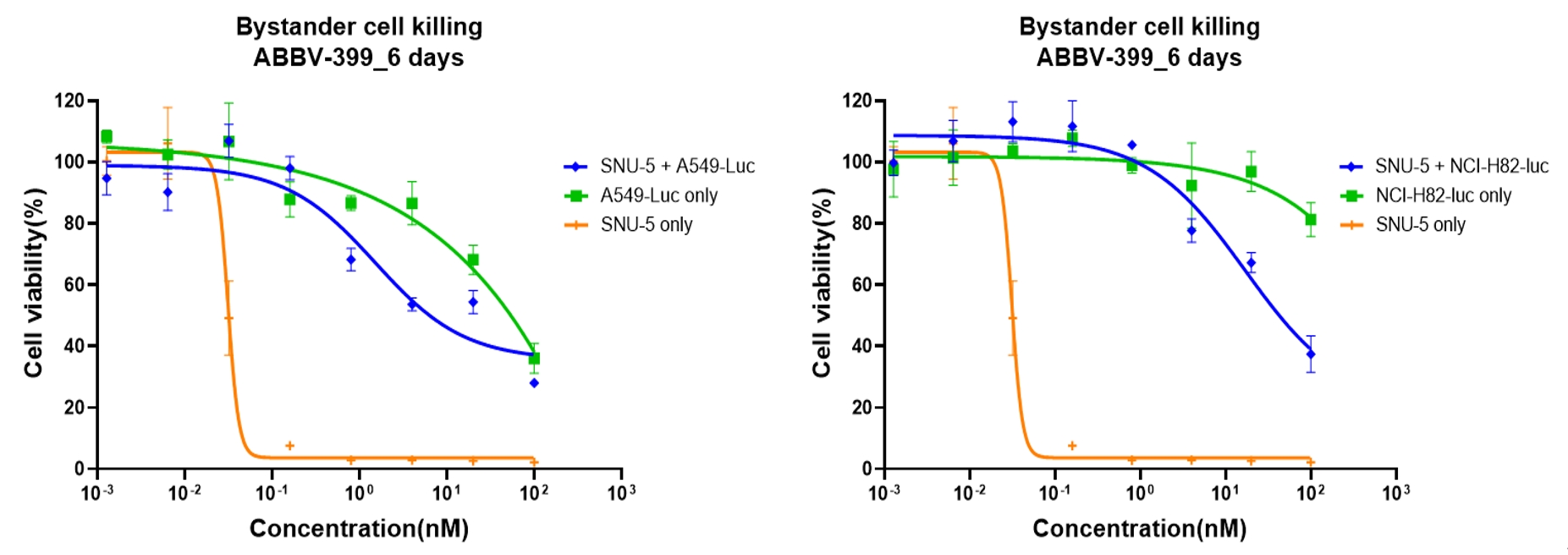
Figure 9. In Vitro Bystander Killing Effect of ABBV-399
GemPharmatech’s ADC evaluation platform provides a comprehensive toolkit for the mechanistic characterization of ADCs, from antigen profiling and binding affinity to internalization efficiency, cytotoxicity and bystander effects. These insights enable more informed decision-making in ADC design and development, supporting the translation of promising candidates into successful clinical therapies.
Contact us at sales@gempharmatech.com to learn how we can support your ADC program.
Reference
1. Zhiwen Fu et al.nature.2022
2. Ogitani et al. Cancer Sci.2016


