Weight Loss of 10% Within 2 Weeks? Evaluating Amylin Analogs in Diet-Induced Obese (DIO) Rat Model

Amylin, a 37-amino-acid peptide hormone co-secreted with insulin by pancreatic β-cells, activates calcitonin receptor complexes (CT/RAMPs) in the nucleus tractus solitarius (NTS) of the hypothalamus to suppress appetite and delay gastric emptying by approximately 50%, thereby enhancing satiety [1]. Physiologically, amylin antagonizes glucagon secretion and reduces hepatic gluconeogenesis, synergizing with insulin to maintain glucose homeostasis [2]. In metabolic disorders (e.g., type 2 diabetes and obesity), amylin deficiency exacerbates uncontrolled feeding and metabolic dysregulation [3], establishing it as a pivotal therapeutic target for obesity.
Cagrilintide represents a third-generation advance in anti-obesity pharmacotherapy
Cagrilintide, a long-acting amylin analog, demonstrates transformative potential in obesity treatment. Its core therapeutic value stems from a dual mechanism: robust activation of amylin receptors (AMY3) in hypothalamic satiety centers to suppress appetite, coupled with delayed gastric emptying. Clinical trials confirm that monotherapy (4.5 mg weekly subcutaneous injection) reduces body weight by 10.8% in obese patients (BMI ≥30) over 26 weeks, significantly outperforming placebo [4]. Studies reveal species-specific effects: amylin analogs induce sustained weight loss in rats but exhibit weaker anorectic effects in mice [5]. Leveraging this divergence, GemPharmatech employed a diet-induced obese (DIO) rat model (12-week high-fat diet [HFD] induction) to test a 4-week intervention. Cohorts received either cagrilintide monotherapy or cagrilintide/semaglutide combination therapy. Body composition (lean/fat mass) and weight were longitudinally monitored via magnetic resonance imaging (MRI) at baseline, week 2, and week 4.
By precisely mimicking amylin’s physiology, cagrilintide achieves rapid weight loss (~10% in 2 weeks monotherapy; ~20% in combination). Emerging combinatorial approaches (e.g., CagriSema) may pioneer reformed paradigms for metabolic disease management.
Testing cagrilintide efficacy in DIO mouse model
In contrast to its efficacy in rat models, cagrilintide lacked therapeutic response in mice. Diet-induced obese (DIO) mice (established via high-fat diet [HFD]) were subjected to 4-week interventions: cagrilintide monotherapy, semaglutide monotherapy, or cagrilintide/semaglutide combination therapy. Longitudinal monitoring of body weight and composition revealed no significant reduction in body weight or fat mass with cagrilintide monotherapy.
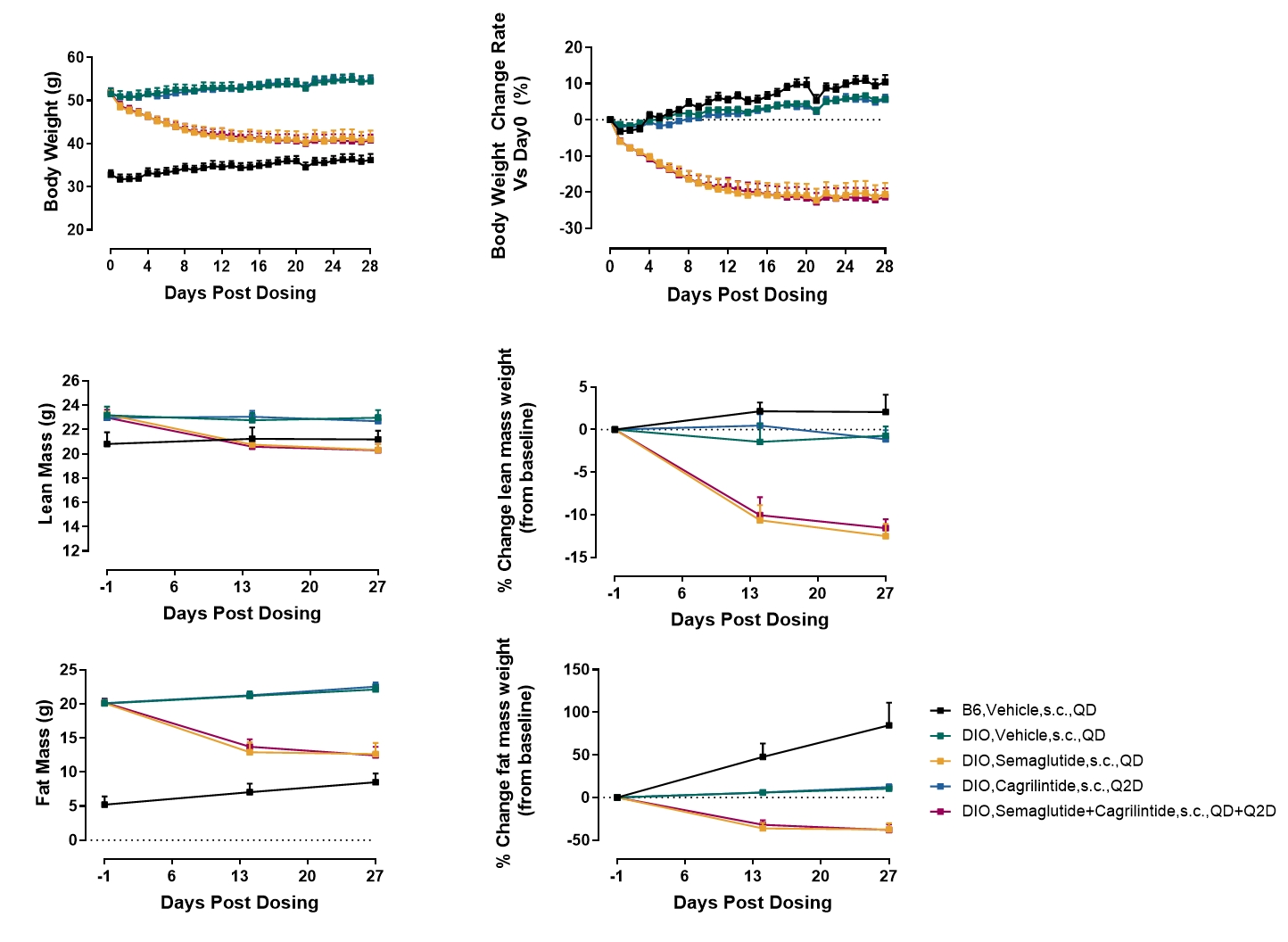
Figure 1 Exposure to cagrilintide failed to reduce body weight or fat mass in diet-induced obese (DIO) mice. Data are presented as Mean±SEM.
Testing cagrilintide efficacy in the DIO rat model
Study design (rat DIO model):

Key findings:
In contrast to the mouse model, cagrilintide monotherapy reduced body weight by 9.5%±0.7 at week 2 in the DIO rat model. Combination therapy with semaglutide further enhanced weight reduction to 18.4% ± 0.7%.

Figure 2 Exposure to cagrilintide reduced body weight loss and combination treatment of cagrilintide and semaglutide partially amplified semaglutide’s association with body weight loss in the DIO rat model. Data are presented as Mean±SEM.
References:
[1] Cell Mol Life Sci. 2012 Jun;69(12):1947-65.
[2] Clin Endocrinol (Oxf). 1996 Mar;44(3):311-6.
[3] Physiol Rev. 2011 Jul;91(3):795-826.
[4] Lancet. 2021 Dec 11;398(10317):2160-2172.
[5] bioRxiv [Preprint]. 2025 Jan 15:2025.01.13.632726.


