Hepatitis B (HBV) is the most common liver infection in the world. According to epidemiological studies of disease progression models from 151 countries in 2022, an estimated 257.5 million people worldwide were infected with the HBV and the global prevalence rate was as high as 3.2%.[1] In the U.S. alone, there were 13,800 estimated acute HBV infections in 2022, and almost 17,000 cases of newly reported chronic HBV. To better study and develop treatments for this disease, more novel mouse models with humanized livers are being developed, and strains such as the NCG Fah KO from GemPharmatech have proven to be critical in preclinical studies.
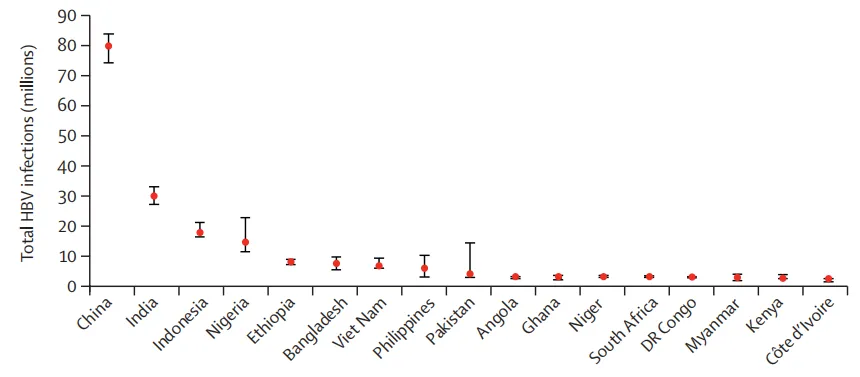
Figure 1. Number of HBV infections in various countries[1]
The hepatitis B virus is a specific hepatotropic DNA virus that exclusively infects humans and some non-human primates. Upon entering hepatocytes, the hepatitis B virus forms covalently closed circular DNA (cccDNA) within the cell nucleus and uses it as a template for viral replication and propagation.
Due to its relatively long half-life, cccDNA is difficult to completely remove from the body. Current clinical treatment drugs mostly consist of nucleosides/nucleotides or interferons, but none of these can eliminate cccDNA entirely. The complete elimination of hepatitis B virus hinges on the natural depletion of cccDNA during treatment, which may render a clinical cure unattainable.
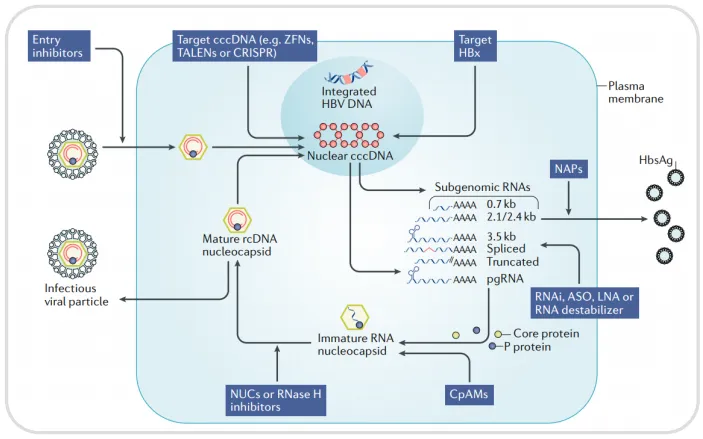
Figure 2. Life cycle of the hepatitis B virus and novel therapeutic interventions
Novel therapeutic drugs target the entire life cycle of the hepatitis B virus, including viral entry into hepatocytes, HBx protein, cccDNA formation, pgRNA, HBsAg formation and secretion. Some therapies also directly target the host.
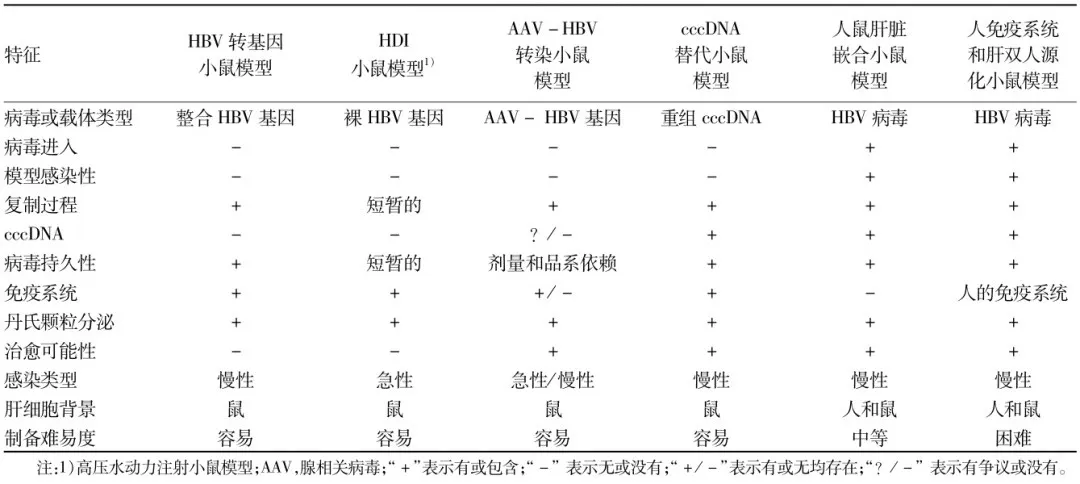
Figure 3. Table with characteristics of HBV infected mouse models[2], (shown in original Chinese)
For the characteristics of a particular model, please contact us at sales@gempharmatech.com.
GemPharmatech has independently developed mouse models with humanized livers. Among them is a model of spontaneous liver injury (NCG Fah KO), in which the engraftment rate of human hepatocytes can reach over 80%. Not only can this model be used to evaluate the efficacy of hepatitis-related drugs, but it can also be used to assess the hepatotoxicity of other drugs, evaluate efficacy of hepatocyte/gene-targeted drugs, and study MASH mechanisms.
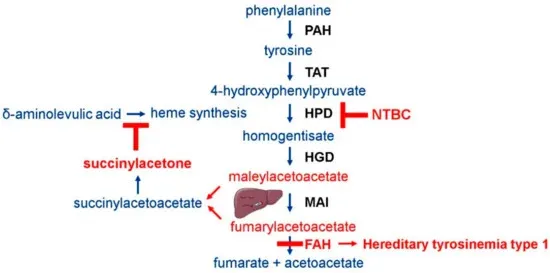
Figure 4. Principles of liver injury in NCG Fah KO[3]
NCG Fah KO mice require Nitisinone (NTBC) supplementation in their drinking water to survive. Upon NTBC withdrawal, these mice develop liver injury and have a lifespan of approximately 4 weeks.
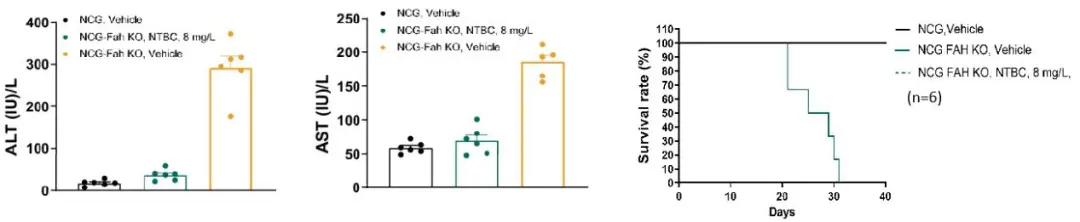
Figure 5. Survival rates of NCG Fah KO mice under different conditions
Ten weeks after receiving human hepatocyte transplantation, NCG Fah KO mice may exhibit human albumin in their serum as high as 10mg/mL, and their liver metabolic enzymes and related proteins are expressed normally.
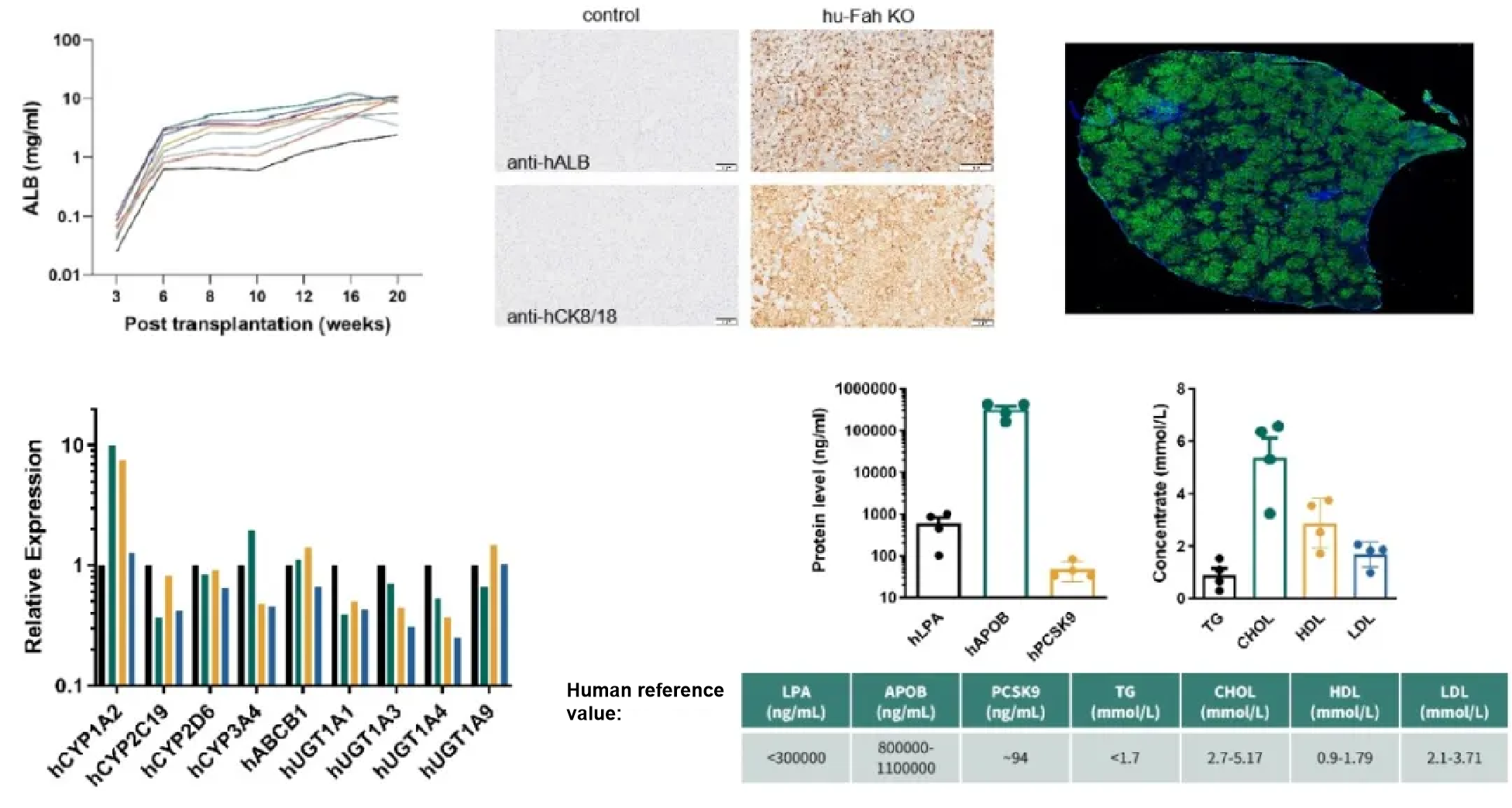
Figure 6. Characteristics of NCG Fah KO mice after human hepatocyte transplantation
In addition to the mouse models mentioned above, GemPharmatech has also developed inducible an inducible liver injury NCG model. If you have any questions or would like to learn more, please do not hesitate to reach out to us at: sales@gempharmatech.com
[1] Polaris Observatory Collaborators. (2023). Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterology & Hepatology, 8(10), 879-907. https://doi.org/10.1016/S2468-1253(23)00197-8
[2] Yang, W., Miao, Z., Song, X., & Yin, M. (2021). Research advances in animal models of hepatitis B virus infection. Journal of Clinal Hepatology, 37(5), 999-1005. https://doi.org/10.3969/j.issn.1001-5256.2021.05.002
[3] Colemonts-Vroninks, H., Neuckermans, J., Marcelis, L., Claes, P., Branson, S., Casimir, G., Goyens, P., Martens, G. A., Vanhaecke, T., & De Kock, J. (2020). Oxidative Stress, Glutathione Metabolism, and Liver Regeneration Pathways Are Activated in Hereditary Tyrosinemia Type 1 Mice upon Short-Term Nitisinone Discontinuation. Genes (Basel), 12(1), 3. https://doi.org/10.3390/genes12010003


