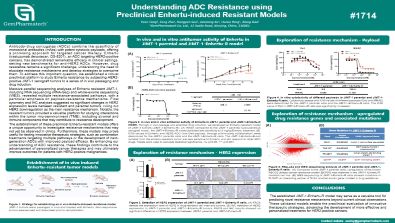
Understanding ADC Resistance using Preclinical Enhertu-induced Resistant Models
Antibody-drug conjugates (ADCs) combine the specificity of monoclonal antibodies (mAbs) with potent cytotoxic payloads, offering a promising approach for targeted cancer therapy. Enhertu (trastuzumab deruxtecan, DS-8201), an ADC targeting HER2-positive cancers, has demonstrated remarkable efficacy in clinical settings, setting new benchmarks for anti-HER2 ADCs. However, drug resistance remains a significant challenge, underscoring the need to elucidate resistance mechanisms and develop strategies to overcome them. To address this important question, we established a robust preclinical platform to study Enhertu resistance by subjecting HER2-positive JIMT-1 xenograft tumors to a series of in vivo passaging and drug induction.
Download
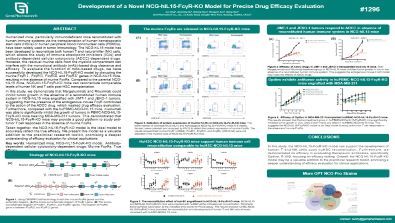
Development of a Novel NCG-hIL15-FcγR-KO Model for Precise Drug Efficacy Evaluation
Humanized mice, particularly immunodeficient mice reconstituted with human immune systems via the transplantation of human hematopoietic stem cells (HSCs) or human peripheral blood mononuclear cells (PBMCs), have been widely used in tumor immunology. The NCG-hIL15 model has been developed to reconstitute both human T and natural killer (NK) cells, which allows the study of immune checkpoint inhibitors (ICIs) and antibody-dependent cellular cytotoxicity (ADCC)-dependent drugs. However, the residual murine cells from the myeloid compartment can interfere with the monoclonal antibody (mAb)-based drug clearance and efficacy. To evaluate the function of mAb-based drugs, we have successfully developed the NCG-hIL15-FcγR-KO model by disrupting the murine FcγRⅠ, FcγRⅡ, FcγRⅢ, and FcγRⅣ genes in NCG-hIL15 mice, resulting in the absence of murine FcγRs. Compared to the parental NCG-hIL15 mice, NCG-hIL15-FcγR-KO mice can reconstitute comparable levels of human NK and T cells post-HSC transplantation.
Download
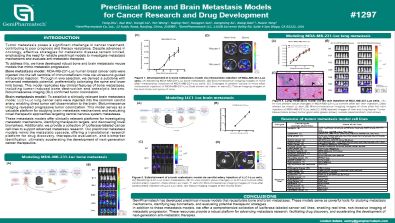
Preclinical Bone and Brain Metastasis Models for Cancer Research and Drug Development
Tumor metastasis poses a significant challenge in cancer treatment, contributing to poor prognosis and therapy resistance. Despite advances in oncology, effective strategies for metastatic disease remain limited, emphasizing the need for reliable preclinical models to investigate metastasis mechanisms and evaluate anti-metastatic therapies. To address this, we have developed robust bone and brain metastasis mouse models that mimic metastatic progression.
Download
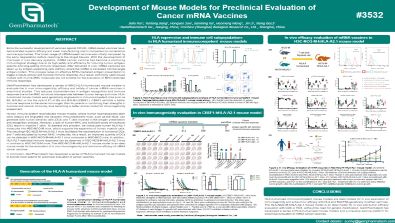
Development of Mouse Models for Preclinical Evaluation of Cancer mRNA Vaccines
Since the successful development of vaccines against COVID, mRNA-based vaccines have demonstrated superior efficacy and lower manufacturing cost in comparison to conventional vaccine approaches. The broad usage of mRNA-based vaccine was initially hampered by the early degradation before reaching to the target tissues. With the development of improved in vivo delivery systems, mRNA cancer vaccine has become a promising immunological strategy due to its high safety and efficiency for inducing tumour antigen-specific and long-lasting immune responses. After delivered in vivo, mRNA vaccines are taken up by antigen-presenting cells (APCs), where the mRNA is translated into the target antigen protein. This process relies on effective MHC-mediated antigen presentation to trigger a robust cellular and humoral immune response. As a result, commonly used mouse models with murine MHC molecules are not suitable for the evaluation of MHC-restricted immune responses.
Download
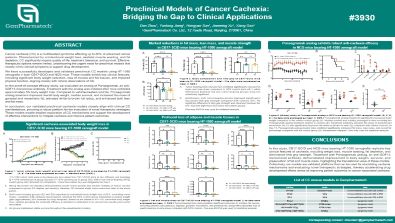
Preclinical Models of Cancer Cachexia: Bridging the Gap to Clinical Applications
Cancer cachexia (CC) is a multifaceted syndrome affecting up to 80% of advanced cancer patients. Characterized by unintentional weight loss, skeletal muscle wasting, and fat depletion, CC significantly impairs quality of life, treatment tolerance, and survival. Effective therapeutic options remain limited, underscoring the urgent need for preclinical models that closely mimic clinical symptoms to support drug development.
Download
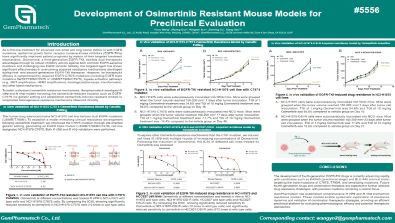
Development of Osimertinib Resistant Mouse Models for Preclinical Evaluation
As a first-line treatment for advanced non-small cell lung cancer (NSCLC) with EGFR mutations, epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) have significantly improved patient prognosis by means of their targeted treatment mechanisms. Osimertinib, a third-generation EGFR-TKI, exhibits dual therapeutic advantages through its robust inhibitory activity against both common EGFR-sensitive mutations and challenging rare EGFR variants. Notably, this targeted agent has shown significant effectiveness in overcoming acquired resistance mechanisms developed during first- and second-generation EGFR-TKI therapies. However, its therapeutic efficacy is compromised by acquired EGFR-C797S mutations (including EGFR triple mutations Del19/T790M/C797S or L858R/T790M/C797S), bypass activation pathways (e.g., MET amplification, HER2 amplification), histological/phenotypic transformation, and other latent mechanisms.
Download
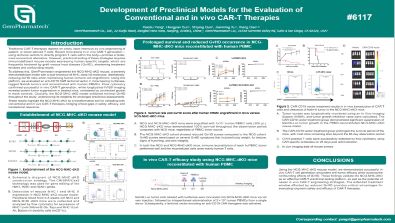
Development of Preclinical Models for the Evaluation of Conventional and in vivo CAR-T Therapies
Traditional CAR-T therapies depend on costly, labor-intensive ex vivo engineering of patient- or donor-derived T cells. Recent innovations in in vivo CAR-T generation—using lentiviral vectors to directly program T cells within the body—promise a faster, more economical alternative. However, preclinical testing of these strategies requires immunodeficient mouse models expressing human-specific targets, which are frequently hindered by graft-versus-host disease (GvHD), shortening treatment windows and confounding results.
Download
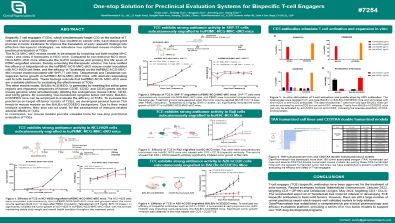
One-stop Solution for Preclinical Evaluation Systems for Bispecific T-cell Engagers
Bispecific T cell engagers (TCEs), which simultaneously target CD3 on the surface of T cells and a tumor-associated antigen (TAA) located on cancer cells, have shown great promise in tumor treatment.To improve the translation of early research findings into effective therapeutic strategies, we introduce two optimised mouse models for preclinical evaluation of TCEs.
Download
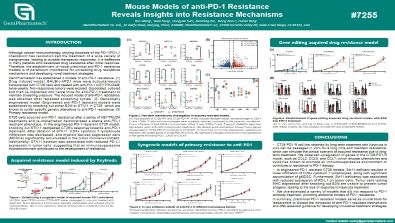
Mouse Models of anti-PD-1 Resistance Reveals Insights into Resistance Mechanisms
Although cancer immunotherapy utilizing blockade of the PD-1/PD-L1 checkpoint has revolutionized the treatment of a wide variety of malignancies, leading to durable therapeutic responses, it is ineffective in many patients who developed drug resistance after initial response. Therefore, the establishment of robust preclinical anti-PD-1 resistance models is of paramount importance for unraveling drug resistance mechanisms and developing novel treatment strategies.
Download


