Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects millions of individuals worldwide. This debilitating condition primarily targets the joints, causing pain, swelling, morning stiffness, and, if left uncontrolled, irreversible damage. With a complex interplay of genetic, environmental, and immunological factors, RA presents a significant challenge to both patients and healthcare providers.
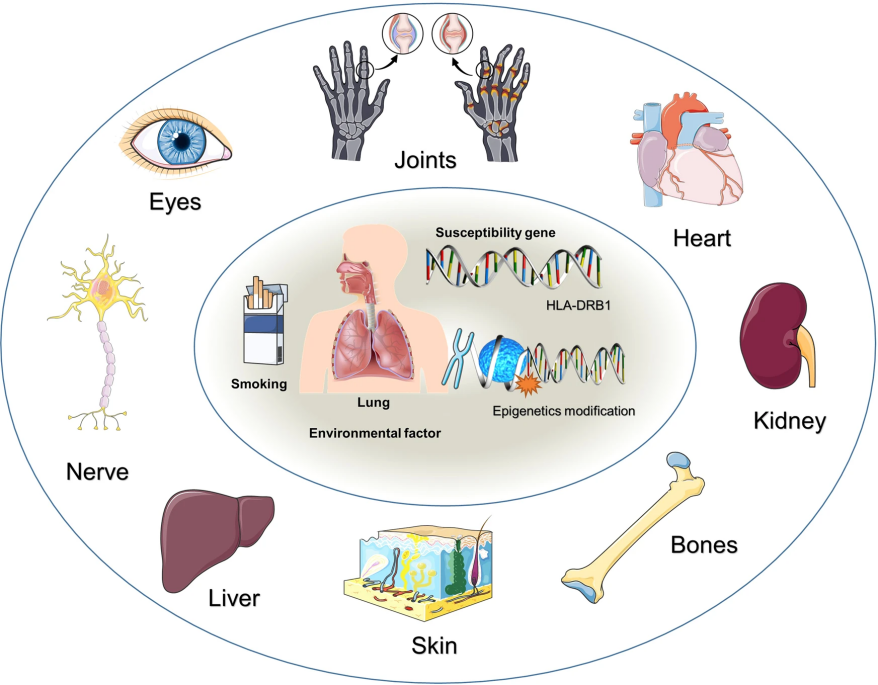
Figure 1. Risk factors and systemic complications of RA[1]
RA typically appears in middle age, with symptoms developing gradually over weeks or months. The prevalence of RA rises with age, and the number of female patients is three to five times that of male patients[2]. The progression involves three stages: a non-specific inflammatory stage, a chronic inflammatory stage, and a tissue damage stage mediated by cytokines such as IL-1, IL-6, and TNF-α. Within the joints, RA triggers an inflammatory response resulting in the infiltration of immune cells, predominantly T cells and B cells. These cells release cytokines that perpetuate the inflammatory cascade, attracting more immune cells and exacerbating joint damage. Over time, chronic inflammation leads to the formation of a pannus, an abnormal layer of tissue that erodes cartilage and bone. The destruction of these supportive structures further compromises joint integrity, leading to deformities and functional limitations.
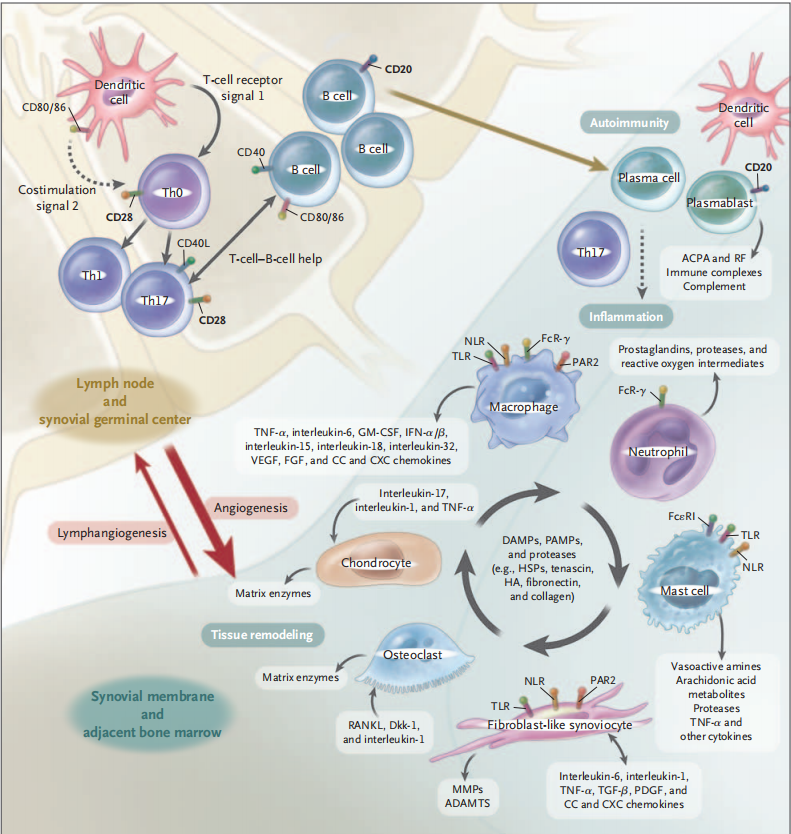
Figure 2. Adaptation and innate immune processes in RA[3]
While there is no cure for RA, several treatment modalities aim to manage symptoms, slow disease progression, and improve patients' quality of life. In the clinic, RA is often treated with nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), and disease-modifying antirheumatic drugs (DMARDs)[1]. The introduction of biologic DMARDs (bDMARDs) targeting TNF-α, IL-6, and IL-1β, among others, and targeted synthetic DMARDs (tsDMARDs) such as Janus kinase (JAK) inhibitors and other small molecule medications over the last decade has ushered in a new era of targeted therapy for RA. These developments provide benefits such as increased precision in treatment and a quicker onset of outcome.
Ongoing research efforts continue to expand the therapeutic options for RA. For instance, the investigational drug fenebrutinib, which can suppress the action of Bruton's tyrosine kinase (BTK), shows promise in effectively treating rheumatoid arthritis patients who have not responded to prior treatments. Researchers are hopeful about the therapeutic potential of BTK inhibitors for RA patients but acknowledge that additional research is necessary. Similarly, JAK inhibitors remain a popular target for RA medicine development, with several JAK inhibitors currently in clinical trials. Ruxolitinib, a selective JAK1/JAK2 inhibitor, for example, has demonstrated benefits in the management of psoriasis and myeloproliferative diseases[1].
The SKG model is a well-established animal model for studying RA. It involves a critical point mutation in the Zap70 gene of mice, where the amino acid at position 163, typically W, is replaced by C. This genetic alteration in SKG mice leads to the spontaneous development of autoimmune arthritis resembling RA, characterized by joint swelling. Another widely used animal model in RA research is the type II collagen-induced arthritis (CIA) mouse model. GemPharmatech has established a reliable CIA model construction system and successfully employed it for preclinical in vivo efficacy evaluation of RA drugs. Additionally, we offer services related to the collagen antibody-induced arthritis (CAIA) mouse model based on customer requirements.
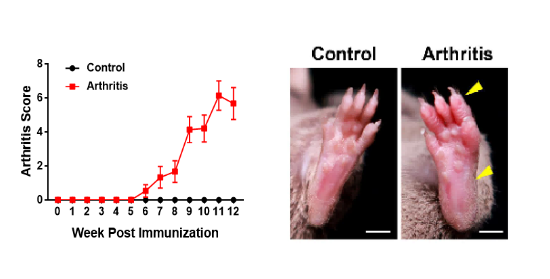
Figure 3. Arthritis scores and morphological changes in CIA mouse models
In the CIA model, type II bovine collagen prepared with complete Freund's adjuvant (CFA) is subcutaneously injected into the tail of mice. Disease scores for the front paws, hind paws, and digits of the mice revealed significantly higher scores in the model group compared to the control group (left figure). By the 10th week, noticeable swelling was observed in the hind paws and digits of the mice in the model group (right figure).
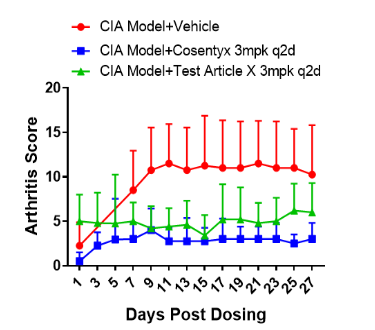
Figure 4. Efficacy evaluation of Anti-IL17A drug in DBA-hIL17A mice
The effectiveness of Cosentyx (secukinumab) and an investigational drug X, targeting hIL17A, was tested on DBA-hIL17A mouse models. Both medications were found to be effective in treating DBA-hIL17A mice.
References:
1. Ding Q, Hu W, Wang R, Yang Q, Zhu M, Li M, Cai J, Rose P, Mao J, Zhu YZ. Signaling pathways in rheumatoid arthritis: implications for targeted therapy. Signal Transduct Target Ther. 2023 Feb 17;8(1):68.
2. Radu AF, Bungau SG. Management of Rheumatoid Arthritis: An Overview. Cells. 2021 Oct 23;10(11):2857.
3. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011 Dec 8;365(23):2205-19.


