Advancing PVR Research: A Novel Transgenic Mouse Model with Spontaneous Disease Phenotype

Proliferative Vitreoretinopathy (PVR) is the formation of scar tissue in the eye after complication of retinal detachment or eye injury. It is one of the most common complications following surgery for rhegmatogenous retinal detachment, with an incidence of approximately 5%–10%. During the course of retinal detachment treatment, once patients progress to severe PVR, the success rate of retinal reattachment surgery decreases markedly, falling to below 50%. The pathological mechanism of this disease is complex, with the core lying in the dedifferentiation, migration, and proliferation of retinal pigment epithelium (RPE) cells which form contractile fibrovascular membranes. These membranes exert traction on the retina, leading to redetachment and ultimately damaging the normal structure and function of the retina.
At present, the therapeutic approaches for PVR are limited, with surgery still serving as the core treatment. However, the postoperative recurrence rate remains high, and no breakthroughs have been achieved in the development of anti-fibrotic, anti-inflammatory, and other related drugs. The fundamental cause of this predicament is the lack of disease models that can accurately simulate clinical pathological features and ensure stable reproducibility. Traditional PVR models are mostly established via intravitreal injection of RPE cells, cytokines, or enzymes, which suffer from drawbacks such as long modeling cycles, significant individual differences and single pathological phenotypes, making them difficult to meet the demands of mechanism research and high-throughput drug screening.
Numerous studies have shown that overexpression of vascular endothelial growth factor A (VEGFA) is a key pathogenic driver of this ophthalmic disease. Under physiological conditions, VEGFA plays an essential role in embryonic development by promoting the formation, growth, and differentiation of new blood vessels. However, pathological overexpression of VEGFA—particularly its splice variant VEGFA165—in ocular tissues leads to sustained proliferation of structurally abnormal blood vessels, increased vascular permeability, and excessive fluid leakage. These changes ultimately contribute to macular edema, retinal detachment, and, in severe cases, irreversible vision loss.
Based on years of experience in transgenic mouse research and development, GemPharmatech has developed a novel B6-Rho-hVEGFA-Tg transgenic mouse model.
Driven by the human rhodopsin promoter, this model overexpresses the coding sequence (CDS) of human VEGFA 165 and develops spontaneous pathological phenotypes without external induction. By 4 weeks of age, it exhibits typical ocular pathological features, including abnormal retinal neovascularization, retinal structural detachment, and formation of proliferative membranes, thereby serving as a novel tool for research on proliferative vitreoretinopathy (PVR) and other related neovascular ophthalmic diseases.
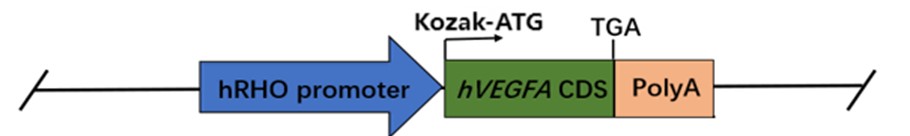
Figure 1: Schematic diagram of the construction strategy for B6-Rho-hVEGFA-Tg mice
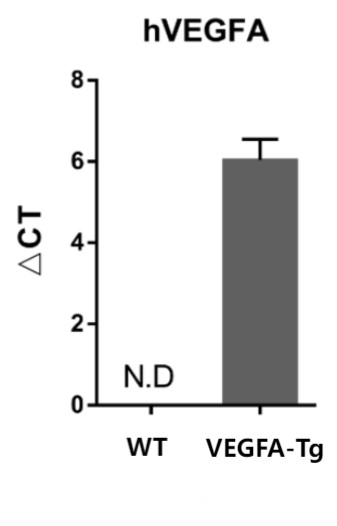
Figure 2: Detection of human VEGFA gene expression levels in mouse eyeballs
By extracting ocular tissues and performing RT-qPCR to verify the expression level of human VEGFA, we found that 8-week-old C57BL/6 control mice showed no hVEGFA expression in the eyeballs, while age-matched B6-Rho-hVEGFA-Tg mice exhibited significant hVEGFA expression. Data are presented as Mean±SD, n=4.
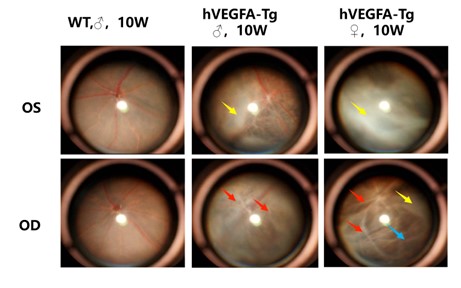
Figure 3: Ophthalmoscopy Findings
Fundus photography of both eyes (OD: right eye, OS: left eye) of mice was performed using an ophthalmoscope. Compared with the eyes of age-matched wild-type control mice, the fundus of B6-Rho-hVEGFA-Tg mice exhibited phenotypes including proliferative membranes (yellow arrows), pigment loss (blue arrows), and vascular sheaths (red arrows).
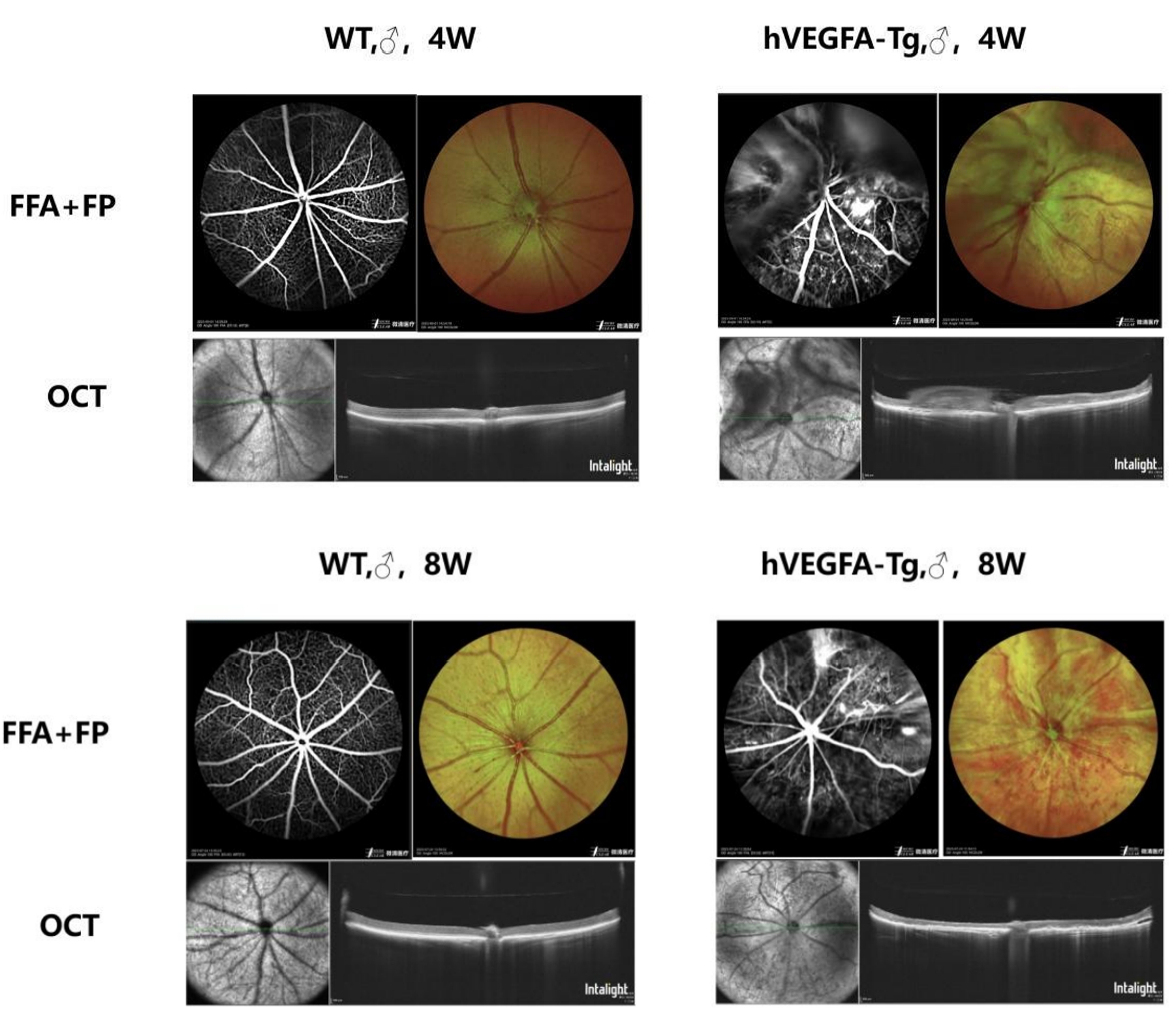
Figure 4: Fundus Imaging and Retinal Morphology Detection of 4-week-old and 8-week-old B6-Rho-hVEGFA-Tg Mice and Wild-type Mice (WT)
Compared with the eyeballs of wild-type control mice of the same week-old, the fundus of B6-Rho-hVEGFA-Tg mice showed retinal pigment loss, tortuous blood vessels, and proliferative membranes above the optic disc. Fluorescein angiography revealed fundus microvascular abnormalities, neovascularization and fluorescent leakage. In addition, the retinal layers were disordered, the neurosensory layer was atrophic and thinned, choroidal neovascularization could be observed in the fundus, proliferative membranes were present above the optic disc, and mild retinal detachment was seen locally.
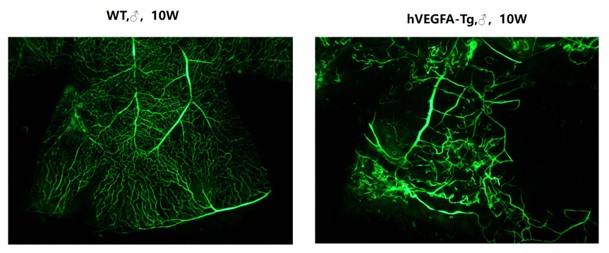
Figure 5: Whole-mount preparation of mouse retina
Compared to the eyeballs of 10-week-old wild-type control mice, retinal flat mounts of age-matched B6-Rho-hVEGFA-Tg mice following FITC-Dextran perfusion showed abnormal vascular proliferation and structural disorganization in retinal vessels.
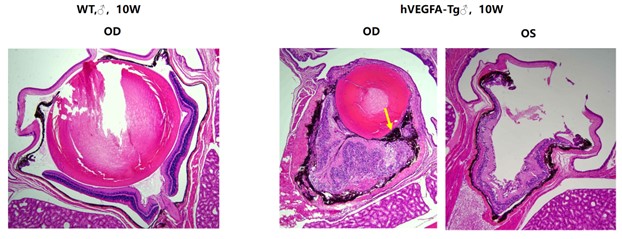
Figure 6: H&E staining of mouse eyeballs
Compared with the eyeballs of 10-week-old wild-type control mice, the retinas of age-matched B6-Rho-hVEGFA-Tg mice exhibited disordered cellular arrangement, indistinct laminar structure, photoreceptor atrophy, and suspected retinal pigment epithelium (RPE) cell proliferation at the site of the epiretinal membrane (indicated by yellow arrows).
Core Advantages of B6-Rho-hVEGFA-Tg Mice:
Compared to traditional induced PVR models, this model features:
1. Spontaneous onset without induction
It avoids the surgical trauma, infection risk, and individual response differences associated with traditional induced models (such as retinal pigment epithelium (RPE) cell injection and enzyme-induced models). This model more closely mimics the natural pathological process of clinical diseases and reduces experimental confounding factors.
2. Strong clinical relevance with human gene overexpression
It overexpresses the human VEGFA gene rather than the endogenous mouse Vegfa gene, which is more consistent with the molecular pathological characteristics of clinical patients. Thus, it provides a more accurate in vivo model for evaluating the efficacy of anti-human VEGFA drugs.
3. Better targeting with retina - specific expression
The target gene is specifically expressed in the retina via the Rho promoter, which avoids damage to visceral organs such as the liver and kidneys caused by systemic overexpression. The experimental animals have high survival rate, making this model suitable for long-term disease course studies.
4. Stable phenotype with high reproducibility
Pathological phenotypes of this mouse are detectable as early as 1 week after birth, with regular disease progression and good intergenerational stability, which can significantly reduce the cost of experimental repetition and render it suitable for high-throughput drug screening.
5. Comprehensive pathological characteristics
It simultaneously exhibits multiple PVR-related pathological features, including abnormal vascular proliferation, retinal structural damage and fibrous proliferation tendency, which can be used for multi-dimensional research on disease mechanisms.
Discover how GemPharmatech can help accelerate your ophthalmic diseases research?
Contact us today and let's start a conversation.


