Non-alcoholic fatty liver disease (NAFLD) has officially been renamed metabolic dysfunction-associated steatotic liver disease (MASLD). This term has been used since the 1980s, but over the last few years it has undergone two renaming controversies. It all stems from the inadequacy of the phrase "non-alcoholic" in its definition.
What is NAFLD, and What is MASLD?
NAFLD, medically speaking, refers to non-alcoholic fatty liver disease. It is a clinical-pathological syndrome characterized by excessive fat deposition in hepatocytes, which is not caused by alcohol or other well-defined liver-damaging factors. As medical research has progressed, scholars discovered that the term "non-alcoholic" is too narrow to encompass the complex etiology and pathogenesis of this disease. In reality, NAFLD is intimately linked to obesity, insulin resistance, dyslipidemia, and other metabolic dysfunctions, which play pivotal roles in its development. Therefore, to better describe this disease, international experts have agreed to rename NAFLD to MASLD.
NASH or MASH
MASLD can be further classified into simple fatty liver disease, metabolic dysfunction-associated steatohepatitis (MASH), and more severe conditions like liver fibrosis, cirrhosis, and even hepatocellular carcinoma. MASH, a severe manifestation of MASLD, is accompanied by significant liver inflammation and fibrosis, posing a significant risk for cardiovascular disease and cirrhosis.
Model Tools
Mouse models are instrumental in MASH research. Multiple mouse modeling methods exist, but a comprehensive comparison shows that the B6-Alms1-del model generates severe metabolic disorders, steatosis, inflammation, and moderate fibrosis, and aligns more closely with the current definition of MASLD.
Models& Features | WD | CDAHFD | HFD+CCl4 | STZ+HFD | B6-Alms1-del* |
Obesity | ++ | - | + | - | +++ |
Hyperglycemia | + | - | +/- | +++ | + |
Hyperinsulinemia | + | - | +/- | - | + |
Hyperlipdemia | ++ | - | + | + | +++ |
Steatosis | +++ | +++ | ++ | + | +++ |
Inflammation | ++ | +++ | +++ | +++ | ++ |
Bllooning | + | ++ | + | +++ | + |
Fibrosis | + | ++ | +++ | ++ | ++ |
Time | 16-28W | 6-12W | 12-16W | 6-12W | Age: ~16Wks |
*Alms1 is an ubiquitous protein that is essential for normal primary ciliary function. Although its specific regulation mechanism has not been fully discovered, the present reports indicate that absence of Alms1 protein will promote nutrient absorption and increase food Intake. Our Alms1 KO model is established based on the specific 11bp sequence of exon 8 of the mouse-derived Alms1 gene deletion. It can cause gene translation termination, forming a mouse model with obesity, diabetes and metabolic dysfunction-associated steatotic liver disease.
Body Weight & Lipid Profile
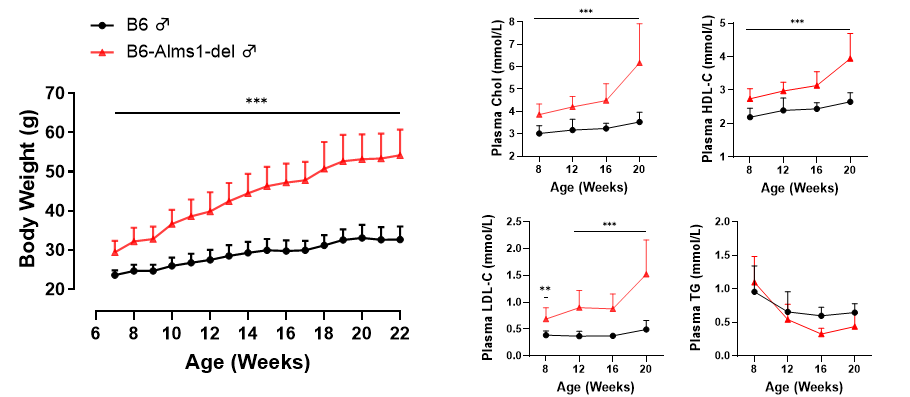
The body weight and four blood lipid indicators of mice were monitored over 22 weeks. The body weight of B6-Alms1-de mice was significantly higher than that of the control group, and the cholesterol level was significantly increased.
Pathological Monitoring
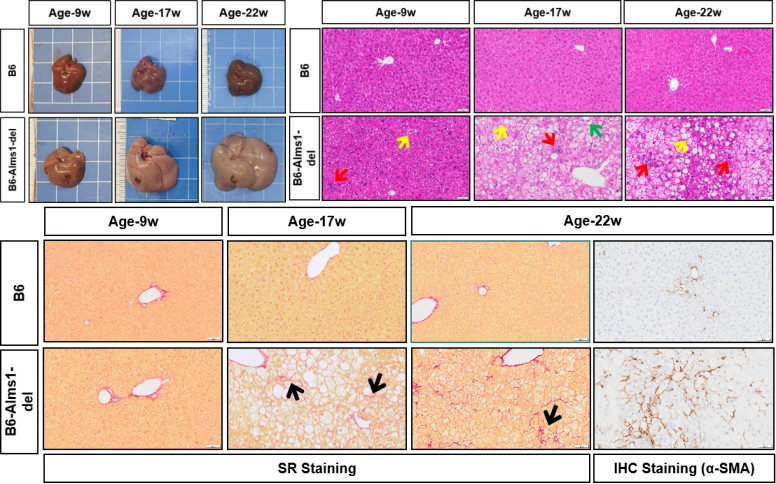
Upon examination of the livers of mice at 9 weeks, 17 weeks, and 22 weeks of age, it was observed that the livers of B6-Alms1-del mice were significantly enlarged compared to the controls, with a pale appearance and signs of fatty degeneration. HE staining showed fatty changes (↑), lobular inflammation (↑), and ballooning degeneration (↑), while Sirius Red and IHC staining confirmed fibrosis.
Pharmacological Validation
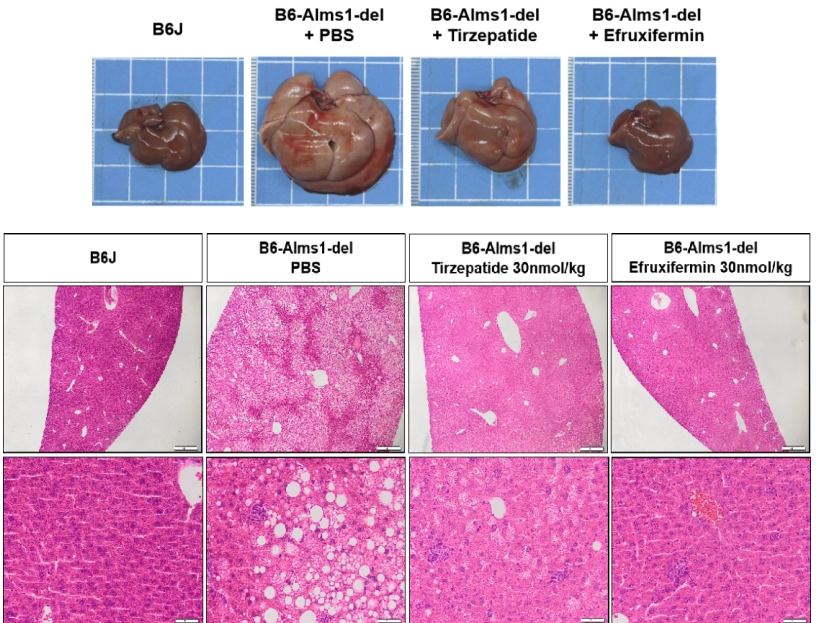
Treatment with 30nmol/kg Tirzepatide or 30nmol/kg Efruxifermin for 6 weeks via subcutaneous injection in 16-week-old male mice significantly improved liver pathology.
Conclusion
NAFLD’s transition to MASLD reflects a deeper understanding of this complex disease. The shift also acknowledges the multifaceted metabolic dysfunctions underlying its pathogenesis, paving the way for more targeted and effective treatments.
To better understand how your research can benefit from our mouse models, please contact us at sales@gempharmatech.com.


