NCG-MHC-dKO mouse model An ideal model for PBMC reconstitution and pharmacodynamic evaluation in the absence of GvHD
June 15, 2024
NCG-MHC-dKO mouse model An ideal model for PBMC reconstitution and pharmacodynamic evaluation in the absence of GvHD
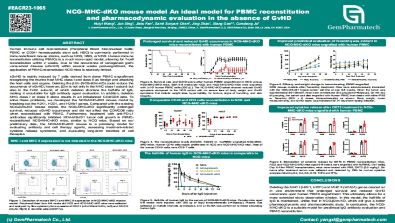
Human immune cell reconstitution (Peripheral Blood Mononuclear Cells; PBMC or CD34+ hematopoietic stem cell; HSC) is commonly performed in immunodeficient mouse strains, such as NCG, NSG, or NOG. Human immune reconstitution utilizing PBMCs is a much more rapid model, allowing for T-cell reconstitution within 2 weeks. Due to the occurrence of xenogeneic graft versus-host disease (xGVHD) within several weeks post-engraftment, the application of PBMC-reconstituted NCG mice is relatively limited.
xGVHD is mainly induced by T cells derived from donor PBMC engraftment recognizing the murine host MHC class I and class II as foreign and attacking the host cells and organs. Deleting the B2m (NCG-B2m-KO) could reduce the occurrence of xGVHD; however, B2m is not only in the MHC class I subunit but also in the FcRn subunit, of which deletion shortens the half-life of IgG, making it unsuitable for IgG antibody agent evaluation. In addition, deletion of MHC class I or class II alone results in an imbalanced CD4/CD8 ratio.
To solve these problems, we developed the NCG-MHC-dKO mouse model by knocking out the H2K1, H2D1, and H2Ab1 genes. Compared with the existing NCG-B2m-KO mouse model, the NCG-MHC-dKO significantly prolonged survival, reduced xGVHD occurrence and did not affect the CD4/CD8 ratio when reconstituted with PBMC. Furthermore, treatment with anti-PD-L1 antibodies significantly inhibited MDA-MB-231 tumor cell growth in PBMC-reconstituted NCG-MHC-dKO mice, similar to NCG mice. Based on our preliminary data, the NCG-MHC-dKO mouse is a promising model for evaluating antibody and cell therapy agents, assessing treatment-related cytokine release syndrome, and evaluating long-term toxicities of cell therapies.
Download
xGVHD is mainly induced by T cells derived from donor PBMC engraftment recognizing the murine host MHC class I and class II as foreign and attacking the host cells and organs. Deleting the B2m (NCG-B2m-KO) could reduce the occurrence of xGVHD; however, B2m is not only in the MHC class I subunit but also in the FcRn subunit, of which deletion shortens the half-life of IgG, making it unsuitable for IgG antibody agent evaluation. In addition, deletion of MHC class I or class II alone results in an imbalanced CD4/CD8 ratio.
To solve these problems, we developed the NCG-MHC-dKO mouse model by knocking out the H2K1, H2D1, and H2Ab1 genes. Compared with the existing NCG-B2m-KO mouse model, the NCG-MHC-dKO significantly prolonged survival, reduced xGVHD occurrence and did not affect the CD4/CD8 ratio when reconstituted with PBMC. Furthermore, treatment with anti-PD-L1 antibodies significantly inhibited MDA-MB-231 tumor cell growth in PBMC-reconstituted NCG-MHC-dKO mice, similar to NCG mice. Based on our preliminary data, the NCG-MHC-dKO mouse is a promising model for evaluating antibody and cell therapy agents, assessing treatment-related cytokine release syndrome, and evaluating long-term toxicities of cell therapies.


